As winter approaches, influenza viruses have entered an increasingly active transmission phase. Recently, the weekly monitoring report from the National Center for Disease Control and Prevention (China CDC) shows that influenza activity has increased in southern provinces of China, with most cases caused by the influenza A (H3N2) strain. Unlike last year's influenza A (H1N1) strain, the general population has lower immunity to H3N2.
Influenza, commonly known as "flu", is a highly contagious acute respiratory infectious disease caused by influenza viruses. It is characterized by strong infectivity, rapid transmission, and the ability to affect both the upper and lower respiratory tracts. Due to its high antigenic variability and rapid transmission capacity, the virus causes seasonal epidemics worldwide every year, posing a serious threat to human health. Influenza viruses are mainly classified into Type A, B, and C. Among them, influenza A viruses, with the widest host range and highest mutation frequency, are the main culprit behind global influenza pandemics.
Q: What are the clinical manifestations of influenza?
The clinical manifestations of influenza include high fever, generalized aches and pains, severe fatigue, accompanied by symptoms such as sore throat, cough, and runny nose, which differ from those of the common cold. It has a short incubation period and acute onset. Susceptible populations include the elderly, young children, pregnant women, individuals with weakened immune systems, and patients with chronic diseases.
Q: How does the influenza virus spread?
The influenza virus is mainly transmitted through droplet transmission and contact transmission. During peak influenza seasons, it is important to take protective measures: frequently wash hands, wear masks, avoid crowded places, and maintain good respiratory hygiene practices.
iGeneTech Influenza Virus Capture Kit
Based on its independently developed TargetSeq® liquid-phase probe capture technology, iGeneTech has developed an influenza virus liquid-phase hybridisation capture kit. The target regions are based on full-length genome sequences of influenza A, B, and C viruses included in the NCBI database over the past 20 years. With reference to 1,250,451 sequences, a total of 33,609 specific probes have been designed.
1. Probe Index Indicators
Species Name | Product Name | Number of Probes | Coverage Rate |
Influenza Virus | Influenza A/B/C Virus Panel | 33609 | 100% |
2. Product Advantages
l Whole-genome coverage, enabling detection of all strains in a single test
l Magnetic bead-based extraction + automated workstation, reducing human error
l Self-developed products throughout the entire process, with no need for additional adaptation
3. Influenza Virus Test Data
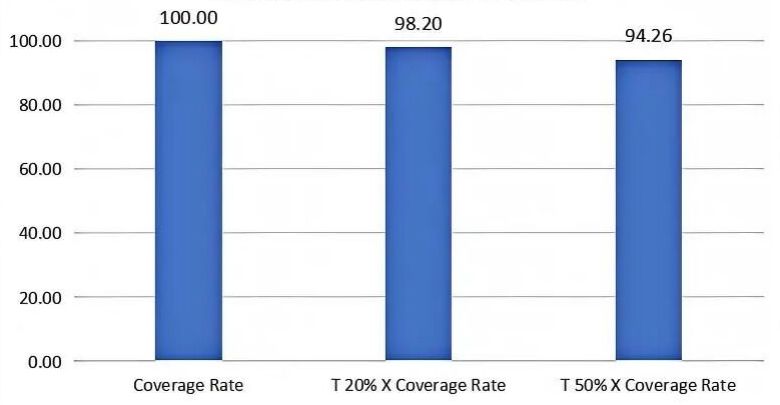
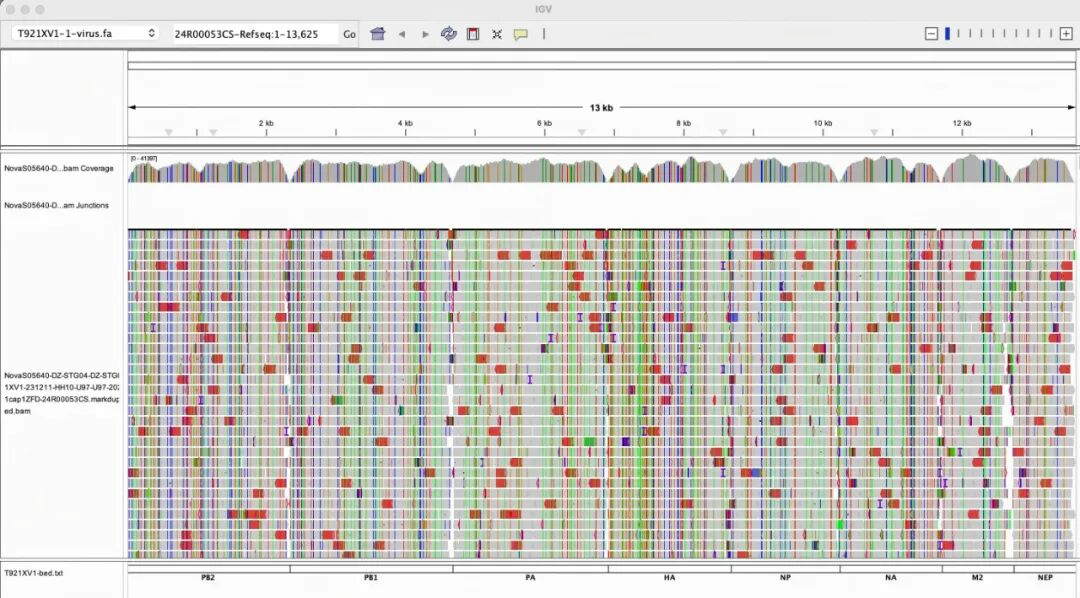
Figure: Performance of Full-Length Capture of Influenza Virus
4. Product Info
Product Name | Speci. | Cat. No |
Influenza A/B/C Virus Panel | 16/96 rxn | PH2000051/PH2000052 |

 CN
CN



