The Jiangsu Provincial Center for Disease Control and Prevention issued a reminder that the Respiratory Syncytial Virus (RSV) has been relatively active in the province recently. This virus is one of the viral pathogens causing acute lower respiratory tract infections in children under 5 years old, and it is even the leading cause of hospitalization due to intrapulmonary infections (such as bronchiolitis and pneumonia) in infants under 1 year old.
The virus is mainly transmitted via droplets and contact. Most infected individuals have mild symptoms, similar to those of a common cold. However, for infants, premature babies, children with congenital cardiopulmonary diseases, or people with weakened immune systems, it can cause severe conditions such as pneumonia, persistent wheezing, or asthma. In most regions of China, the peak epidemic period usually lasts from November to April of the following year. The incubation period of RSV infection is 2-8 days, with an acute onset. Mild symptoms include nasal congestion, runny nose, sneezing, cough, low-grade fever, and sore throat (similar to the common cold); severe symptoms may involve rapid breathing, wheezing, triple concave sign (retraction of the suprasternal fossa, supraclavicular fossa, and intercostal spaces during inhalation), and cyanosis.
Currently, there is no widely available RSV vaccine in China. Immunity after infection is not long-lasting, and repeated infections may occur. Therefore, preventive measures are particularly important. During the peak period of RSV, it is advisable to frequently wash hands, cover the mouth and nose when coughing or sneezing, and thoroughly clean hands before touching infants. Do not take children to crowded and enclosed places such as shopping malls and hospitals to avoid cross-infection.
iGeneTech Respiratory Syncytial Virus (RSV) Capture Kit
To better monitor and diagnose RSV infections, iGeneTech, based on its independently developed TargetSeq® liquid-phase probe capture technology, has launched the Respiratory Syncytial Virus liquid-phase hybridization capture kit. Taking the full-length genome sequences included in the NCBI database over the past 20 years as the target regions, it has referenced 5,438 sequences and designed a total of 5,391 specific probes.
1. Probe Index Indicators
Species Name | Product Name | Number of Probes | Coverage Rate |
Respiratory Syncytial Virus | Human Respiratory Syncytial Virus Panel | 5391 | 100.00% |
2. Product Advantages
01 Whole-genome coverage, enabling detection of all strains in a single test;
02 Magnetic bead-based extraction + automated workstation, reducing human error;
03 Self-developed products throughout the entire process, with no need for additional adaptation.
3. Respiratory Syncytial Virus Test Data
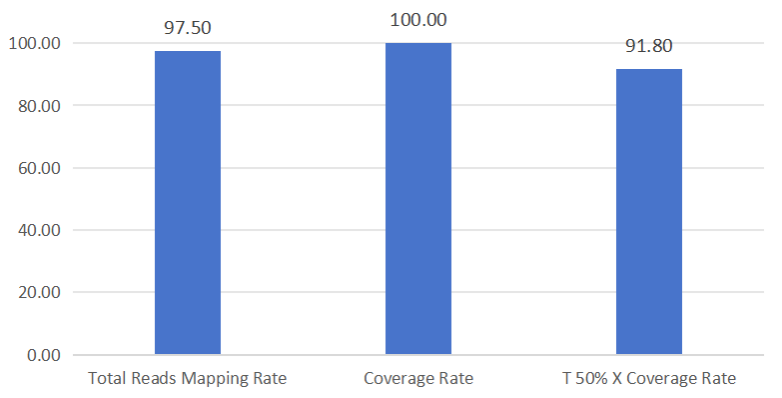
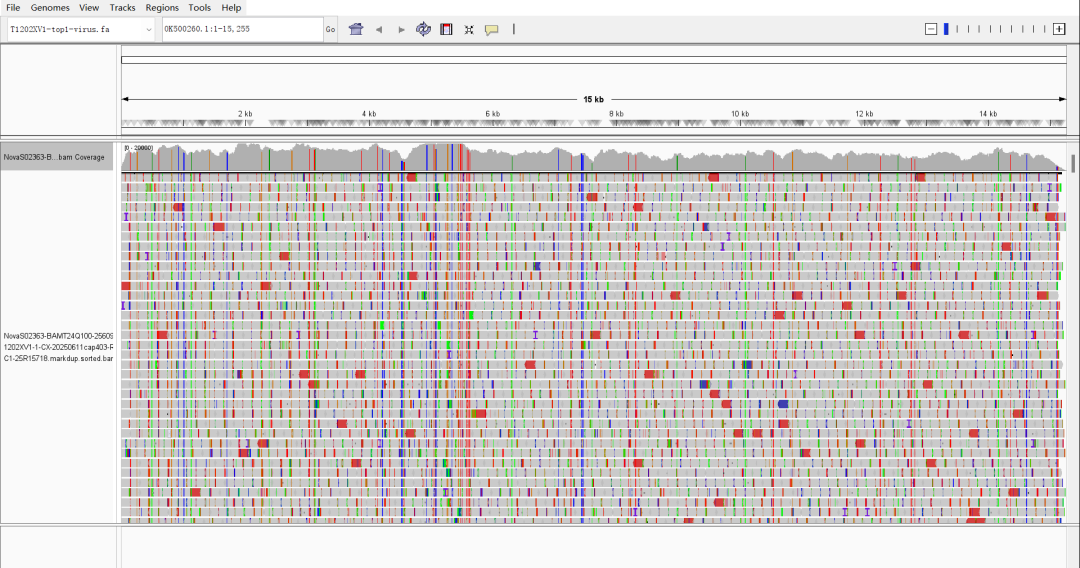
Figure: Full-Length Capture Performance of Respiratory Syncytial Virus
4. Product Info
Product Name | Speci. | Cat. No |
Human Respiratory Syncytial Virus Panel | 16/96 rxn | PH2000701/PH2000702 |

 CN
CN