Since November, the Omicron variant of SARS-CoV-2 (B.1.1.529) has emerged in over 77 countries and regions. According to the World Health Organization, Omicron is spreading at an unprecedented rate. In China, after Tianjin reported an imported Omicron case among quarantined personnel, Guangzhou also announced a confirmed imported Omicron case on December 14.
As an RNA virus, SARS-CoV-2 is highly prone to mutation. The Omicron variant contains over 50 mutations—far more than previous variants—with 10 of these mutations located in the receptor-binding domain (RBD) of the spike protein. These changes may significantly enhance viral transmissibility and human-to-human spread. In South Africa, where Omicron was first identified, infection rates surged rapidly, and the variant spread quickly (Figure 1).
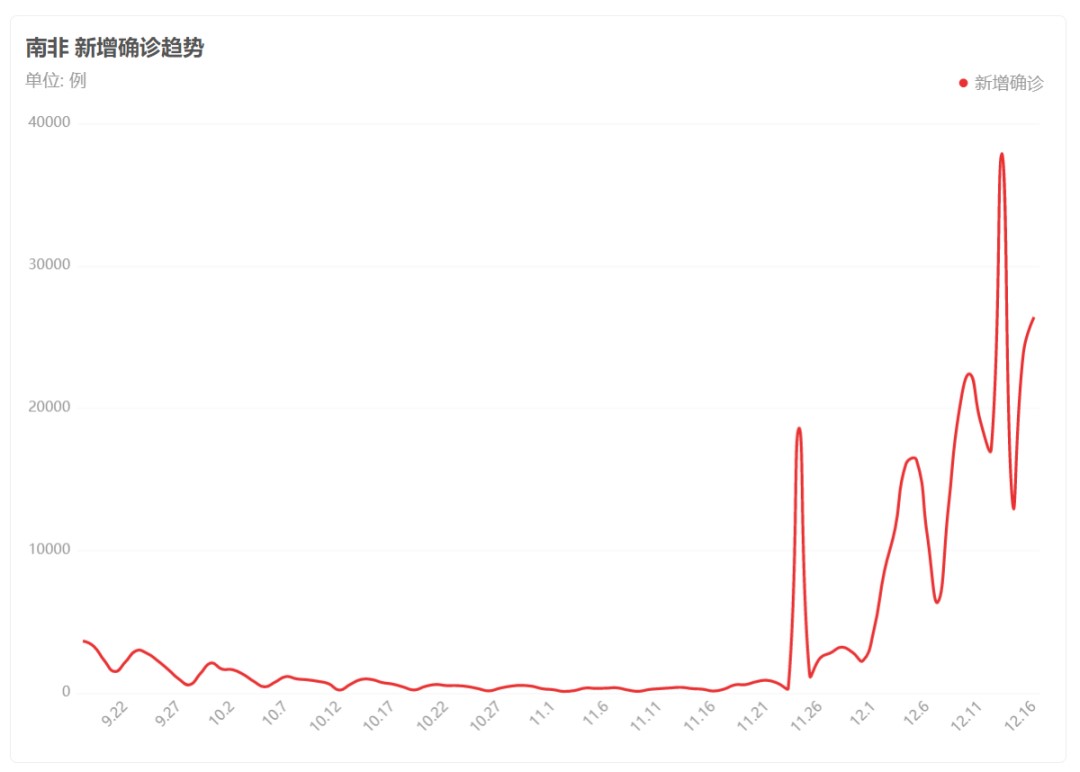
Figure 1. Trend of COVID-19 cases in South Africa
Currently, the full-length SARS-CoV-2 detection commonly used in China is primarily based on multiplex amplicon sequencing technology. However, the rapid evolution of the virus and the emergence of new mutations pose a challenge for PCR-based detection methods and create significant pressure for manufacturers of full-length detection kits. The ability to detect the full genome of the Omicron variant has become a key benchmark for evaluating the performance of COVID-19 test kits.
Upon the release of Omicron's mutation profile, iGeneTech promptly used the industry-standard multiplex primer quality evaluation tool, MFEprimer 3.1, to scientifically assess the performance of its current version of the AI-SARS-CoV-2-Multi Panel (full-length multiplex detection kit) in identifying Omicron-specific mutations. The evaluation confirmed that the kit accurately identifies all major variants, including Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), Gamma (P.1), and Omicron (B.1.1.529).
Building on this, iGeneTech implemented an upgrade to its full-length detection kit based on a primer design strategy for fast-mutating species proposed by co-founder Dr. Wubin Qu. The kit was updated with primers targeting newly emerging Omicron mutations and validated using a SARS-CoV-2 reference standard from Qingliang Gene. Results showed strong amplification at Omicron mutation sites: with a standardized sequencing depth of 1000x, the minimum sequencing depth at mutation sites exceeded 210x, and the average depth reached 1446x (Table 1).
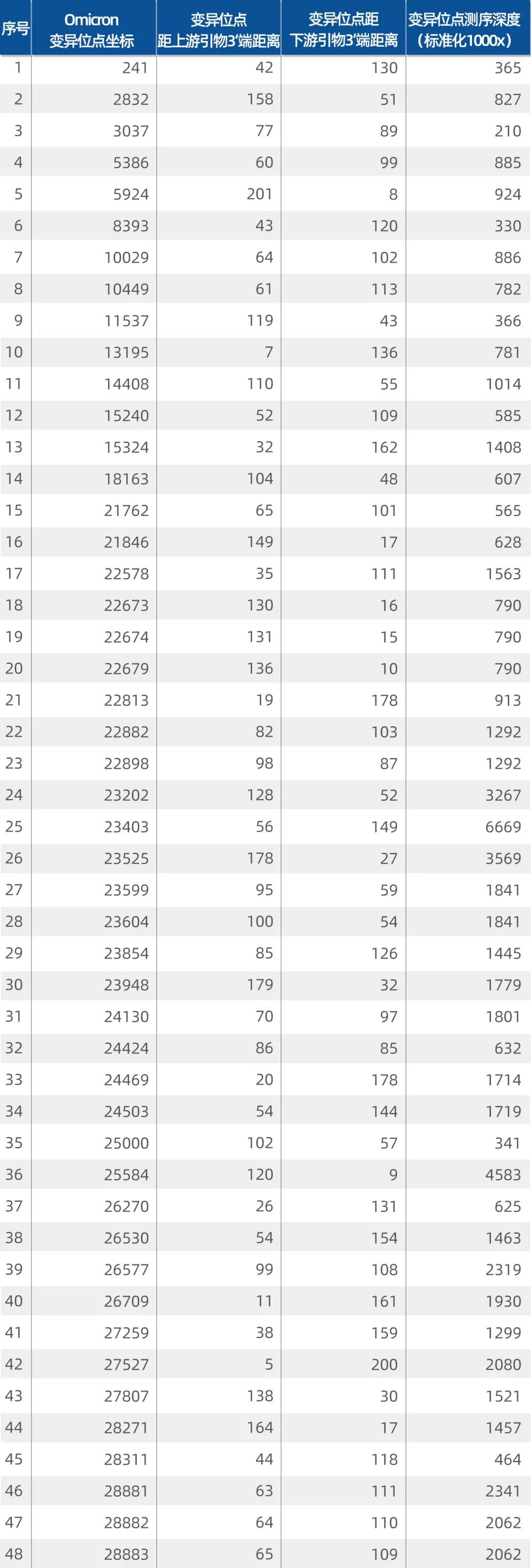
Table 1. Omicron mutation site coordinates and sequencing depth
In addition to optimizing for Omicron-specific mutations, iGeneTech also enhanced the overall performance of the detection kit. Key metrics such as genome coverage and uniformity were significantly improved (Table 2).
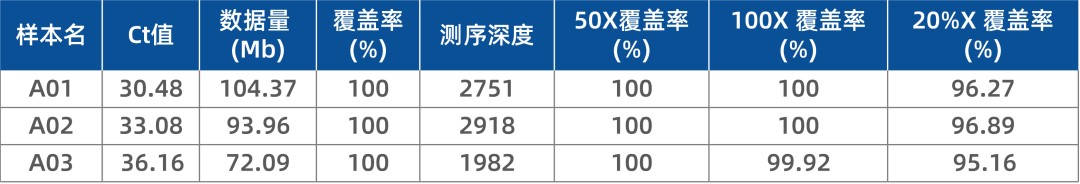
Table 2. Performance data of iGeneTech's full-length SARS-CoV-2 multiplex kit
As the pandemic intensifies overseas and new local cases continue to appear in various parts of China, the pressure to detect all SARS-CoV-2 variants quickly and accurately has increased dramatically. This is not only a societal demand but also the mission iGeneTech has been tirelessly pursuing.
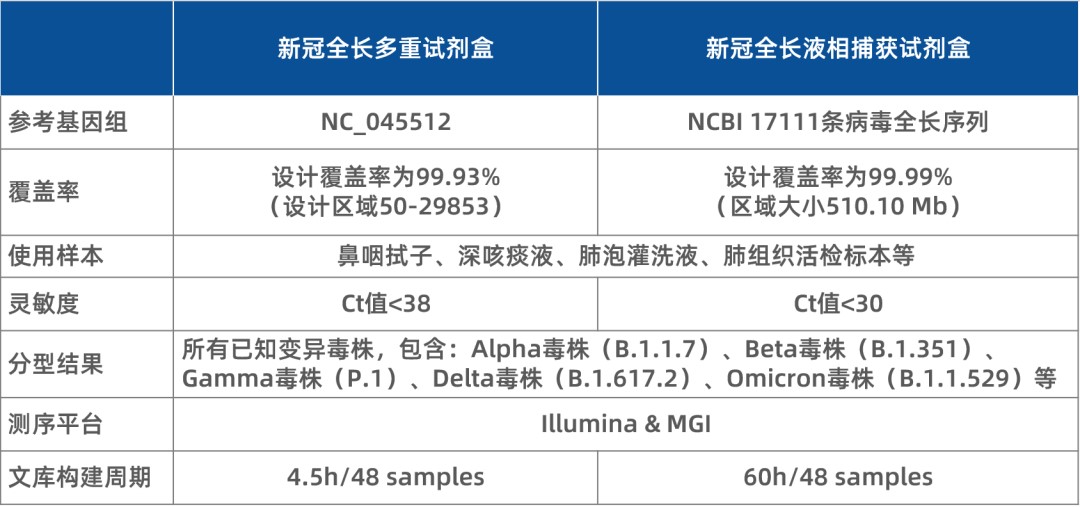
Currently, iGeneTech offers two high-performance full-length detection kits:
Both kits are well-equipped to detect the Omicron variant, and the entire library preparation process for 48 samples can be completed in as little as 4.5 hours. iGeneTech welcomes inquiries and orders from CDC centers and SARS-CoV-2 testing institutions nationwide.
Table 3. Key features of iGeneTech's full-length SARS-CoV-2 detection kits
iGeneTech will continue to closely monitor SARS-CoV-2 mutations and rapidly optimize its products to provide users with the highest quality full-length virus detection services.n services.
About iGeneTech®
iGeneTech is a national high-tech enterprise focused on gene capture technologies. It owns independent intellectual property rights for both probe hybridization and multiplex PCR gene capture platforms. The company is capable of delivering large-scale, high-throughput gene capture-based diagnostics and offers global leading solutions in gene capture technology.
Through years of R&D and industrialization, iGeneTech has developed a comprehensive product line covering all aspects of gene capture, including: customized capture panel development, LIMS systems for labs, full-process automation solutions, data management and interpretation systems, laboratory quality management solutions, ultra-fast NGS sequencing services, and high-throughput sample-based diagnostic services.
iGeneTech has become a global provider of complete gene sequencing solutions, offering fully integrated, highly automated, and intelligent gene testing services to third-party clinical labs, precision medicine centers in hospitals, genetic testing companies, and clinical researchers worldwide.

 CN
CN