Systemic Lupus Erythematosus
Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by abnormal activation of the immune system, which can affect multiple organs and systems throughout the body. Among its manifestations, the most common and severe one is Lupus Nephritis (LN), which occurs in approximately 35%-60% of SLE patients. Symptoms of LN include proteinuria, hematuria, edema, and hypertension; in severe cases, it can progress to renal failure, making it one of the major causes of death in SLE patients. Traditional Chimeric Antigen Receptor T-Cell Therapy (CAR-T cell therapy) has a long treatment cycle and high cost. Moreover, immunosuppressants must be discontinued during CAR-T cell preparation, which may lead to disease exacerbation.
Clinical Breakthrough
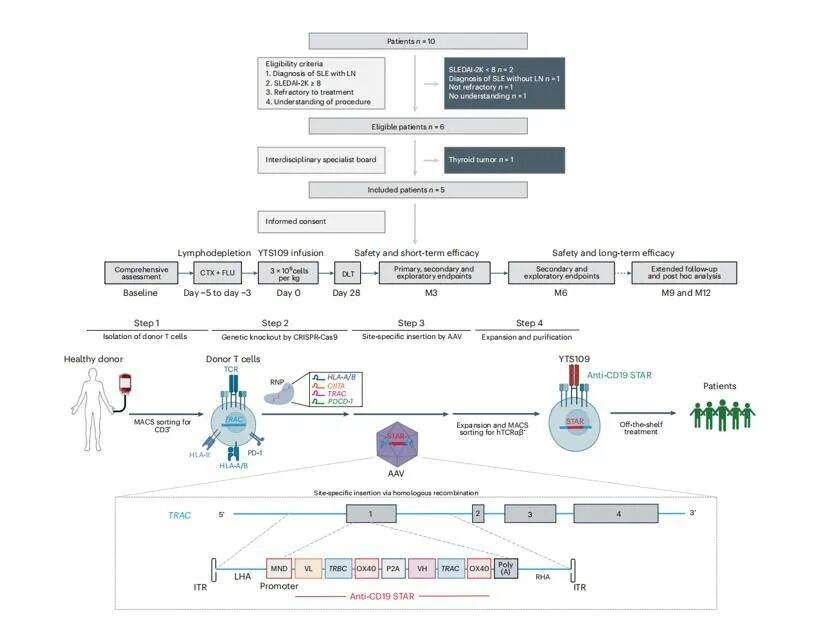
On August 27, 2025, a research team led by Professor Xu Huji from The Second Affiliated Hospital of Naval Medical University (Shanghai Changzheng Hospital), in collaboration with Professor Lin Xin's team from the School of Basic Medical Sciences, Tsinghua University, published a major research achievement on cell therapy for severe SLE complicated by LN in the journal Nature Medicine, with the title "Allogeneic CD19-targeting T cells for treatment-refractory systemic lupus erythematosus: a phase 1 trial".
This study is the first to apply a novel allogeneic cell therapy to the treatment of refractory SLE complicated by LN, achieving a breakthrough in clinical trials. The allogeneic STAR-T cell (YTS109) enables precise immune resetting and renal function repair by efficiently eliminating pathogenic B cells.
Research Methods and Results
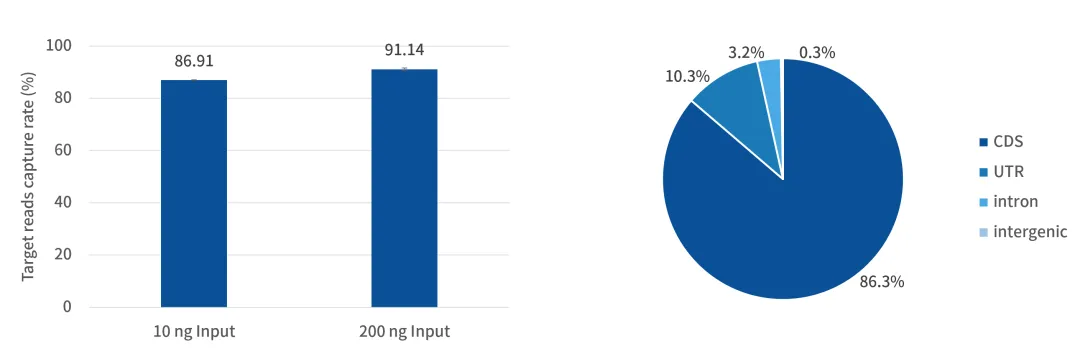
The research team employed CRISPR-Cas9 gene editing, single-cell sequencing, spatial transcriptome sequencing, plasma proteomics, and whole-exome sequencing (using the AIExome® Human WES V3 Panel) to systematically verify the safety, efficacy, and "immune resetting" mechanism of YTS109, providing key evidence for its clinical translation.
The results showed that STAR-T cell therapy significantly improved renal function, with a substantial reduction in 24-hour urinary protein levels. Patients achieved complete renal remission (<500 mg/24h) within 1-2 months. Renal biopsies revealed the resolution of glomerular inflammation, a decrease in immune complex deposition, and improvements in quality of life scores.
iGeneTech’s Privilege to Contribute
iGeneTech was privileged to provide high-quality reagents (AIExome Human Exome Panel V3 Panel) required for exome capture in this study. These reagents helped identify pathogenic genetic variants (such as IRF5, IFNW1, and C2) in patients, assisted in revealing the genetic basis of severe refractory SLE, and were combined with clinical responses and immunological mechanisms to facilitate the understanding of treatment efficacy and individual differences.
Original Link: https://www.nature.com/articles/s41591-025-03899-x
iGeneTech Product Advantages
Relying on its independently developed TargetSeq® Hybrid Capture Sequencing Technology and IGT® Oligo Pools Large-Scale Parallel Synthesis Platform, iGeneTech can quickly provide a wide range of high-quality predefined and customized products. It delivers a comprehensive solution covering the entire process from probe design, Oligo Pools synthesis, and probe preparation to the delivery of probes and capture kits.
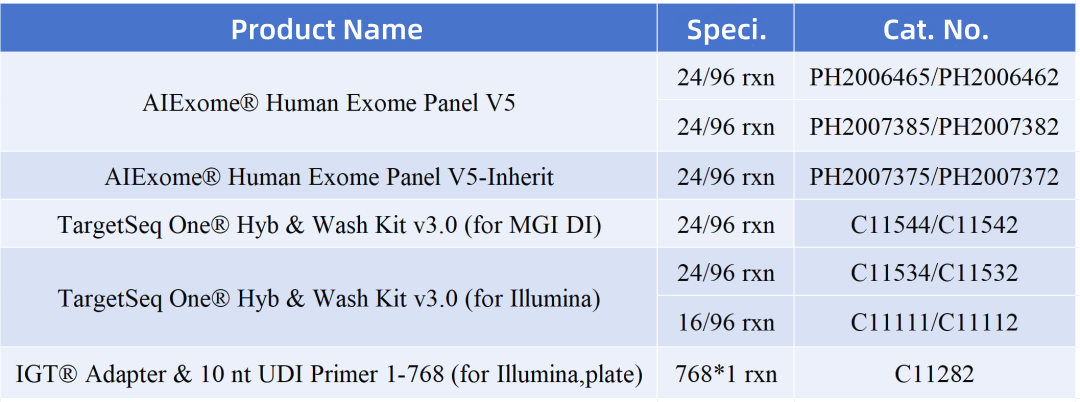
Product Info

 CN
CN