Human leukocyte antigen (HLA), the major histocompatibility complex (MHC) in humans, determines cellular histocompatibility and immune response capabilities to different antigens. HLA testing is closely associated with organ transplantation, bone marrow transplantation, precision medicine, paternity testing, etc., and has increasingly important links to autoimmune diseases, tumor immunity, and infection immunity with ongoing research. Leveraging its decade-long proprietary TargetSeq® hybrid capture sequencing technology and matching reagent solutions, igenetech has developed multiple HLA products to meet diverse customer testing needs. This article shares research applications of iGeneTech's HLA products in high-precision typing and introduces the company's latest HLA product solutions.
Customer Article 1: Identification of Nasopharyngeal Carcinoma-Specific Peptides Using Immunopeptidomics [1]

01 Research Background
The Epstein-Barr virus (EBV) is closely associated with nasopharyngeal carcinoma (NPC). Studies indicate that over 95% of adults have been infected with EBV, and EBV is detected in nearly all NPC cases. T cell-mediated immune responses can target and kill EBV-positive tumor cells, while EBV virus-specific antigens must be presented to T cell surface-specific recognition receptors (TCRs) by HLA class I molecules. Therefore, identifying EBV-derived peptides presented by HLA class I molecules throughout the EBV life cycle is critical [2].
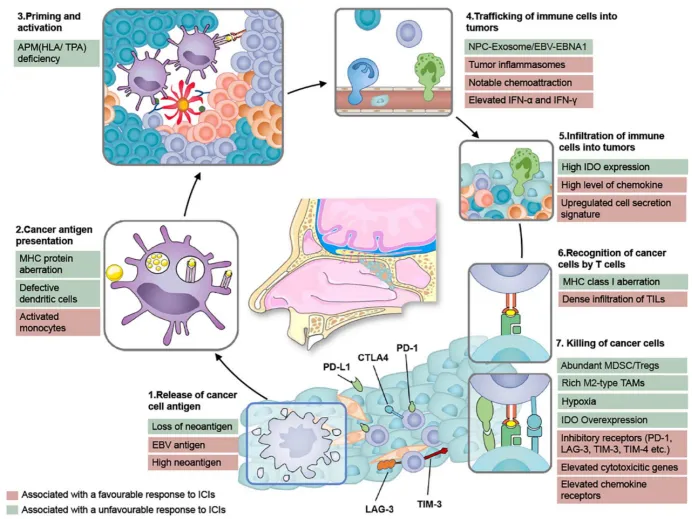
Figure 1. Biological Steps for Achieving Effective Immune Response in Nasopharyngeal Carcinoma [3]
02 Research Methods
Researchers from Professor Miao Xu’s team at the Sun Yat-sen University Cancer Center established EBV-positive nasopharyngeal carcinoma cell lines. They used immunoprecipitation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to isolate and identify EBV lytic-phase specific peptides. Validation was performed using the IFN-γ ELISPOT assay on EBV VCA/NA-IgA-positive healthy donors.
03 Selected Research Results
To validate the immunogenicity of identified EBV peptides in inducing HLA-A11:01- and HLA-A02:01-restricted CD8+ T cell responses, the team utilized iGeneTech’s predefined product, TargetSeq® Human hla panel, for typing. They selected 5 healthy donors carrying the HLA-A02:01 allele and 11 donors with HLA-A11:01. Subsequent IFN-γ ELISPOT assays confirmed the peptides’ immunogenicity, providing new insights into EBV infection surveillance and NPC prevention.
This study demonstrated that highly prevalent HLA class I-restricted EBV peptides in in vitro models can be captured and functionally induce immune responses. This provides evidence for antigenic determinants presented during EBV lytic activation and reactivation, offering novel targets for early diagnosis and treatment of nasopharyngeal carcinoma.
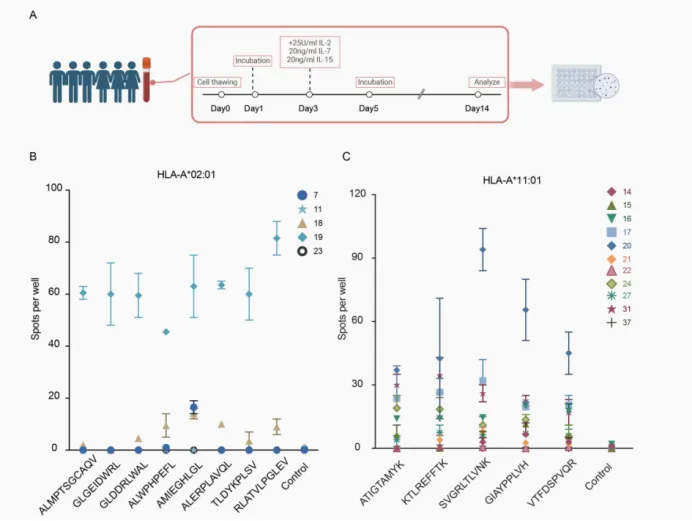
Figure 2. Immunogenicity Validation Protocol for EBV-Positive Healthy Donors with Selected Results Presented
Customer Article 2: Decoding the 3D Chromatin Architecture of Human Primary Monocytes [4]

01 Research Background
Chromatin interactions play a critical role in establishing and maintaining immune cell functions during the development, differentiation, and activation of autoimmune diseases. To date, high-resolution 3D landscapes of primary monocytes in innate immunity remain scarce.
02 Research Methods
Researchers from Zhao Yueran’s team at Shandong University selected peripheral blood from 2 systemic lupus erythematosus (SLE) patients and 2 healthy individuals. They prepared CD14+ monocytes and employed Hi-C, RNA-seq, ATAC-seq, and ChIP-seq for detailed analysis.
03 Selected Research Results
The constructed high-resolution chromatin interaction maps revealed similar landscapes between SLE patients and healthy controls, with significant differences compared to the chromatin structure of THP1 cell lines. Chromatin interactions near HLA-D genes showed high variability among individuals. iGeneTech’s predefined product, TargetSeq® Human HLA Panel, was used in this analysis. Results indicated that high polymorphism in sequences may affect 3D genome structure, thereby influencing the regulation of related gene expression patterns.

Figure 3. Typing Results of Study Individuals Using iGeneTech’s TargetSeq® Human HLA Panel
New Product Launch
01 Product Introduction
Designed with probes targeting full-length sequences of HLA-A, B, C, DRB3/4/5 genes and full exon sequences of HLA-DRB1, DQB1, DPB1, DQA1, DPA1, DOA, DOB, DMA, DMB genes, based on the latest IMGT/HLA database. This product enables 6-digit high-resolution typing, applicable to transplant matching, autoimmune disease research, pharmacogenomics, and other fields.
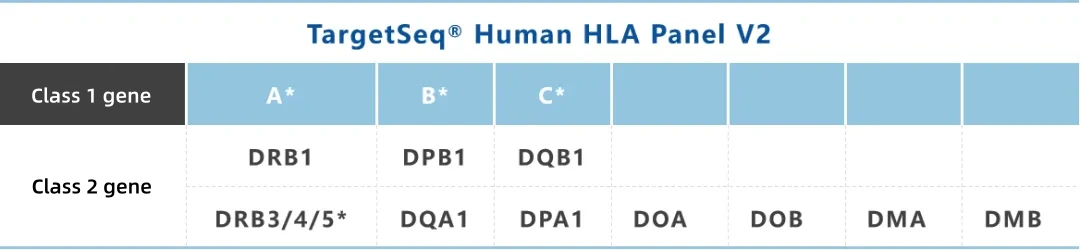
* Genes designed for full-length sequences
Product Design
The major histocompatibility complex (MHC) has two distinct features that make it difficult for pathogens to escape the immune response. First, its polygenic nature includes a variety of MHC class I and II genes. Second, it exhibits high polymorphism, with multiple mutations or alleles present in each gene.

Figure 4. Gene Structure Diagram of Human MHC Molecules [5].
Human MHC class I molecules have three α chain-encoding genes: HLA-A, HLA-B, and HLA-C. Additionally, there are three pairs of MHC class II α and β chain-encoding genes: HLA-DR, HLA-DP, and HLA-DQ. The HLA-DR gene cluster contains an extra gene encoding a β chain, whose product can pair with the DR α chain. This allows the three gene pairs to generate four types of MHC class II molecules. HLA-DM molecules are encoded by DMA and DMB genes, and these subunits catalyze the binding of MHC class II molecules to antigenic peptides. HLA-DO molecules, encoded by DOA and DOB genes, negatively regulate HLA-DM function [5]. Collectively, iGeneTech’s TargetSeq® Human HLA Panel V2 includes 15 immunologically critical and highly polymorphic genes to meet diverse research objectives.
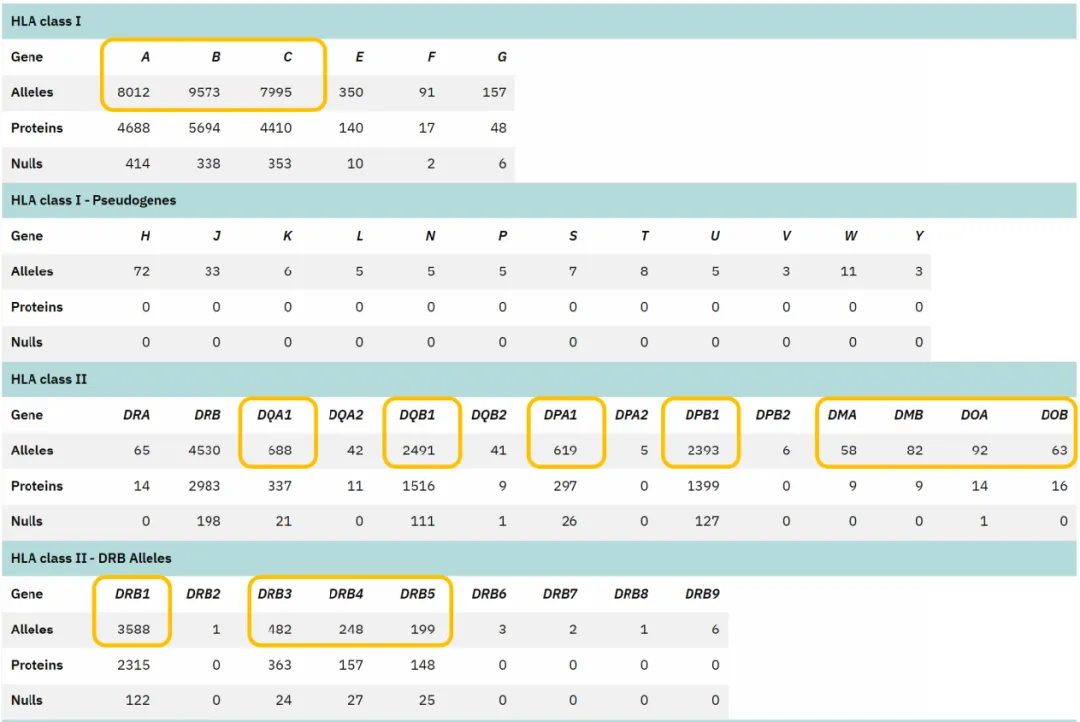
Figure 5. Number of Alleles in the IMGT/HLA Database (Version 3.54), with Genes Included in iGeneTech’s TargetSeq® Human HLA Panel V2 Highlighted in Yellow.
02 Product Features
· Exceptional probe design strategy: Designed using all polymorphic sequences of genes in the IMGT/HLA database.
· High precision: Achieves 6-digit precision typing through integration of NGS high-throughput sequencing and HLA-HD typing software.
· Flexible customization: Supports semi-customization to add additional HLA genes according to specific needs.
03 Typing Performance
Peripheral blood samples were typed using iGeneTech’s TargetSeq® Human HLA Panel V2. The results showed high consistency with HLA-A, B, C, DRB1, and DQB1 typing outcomes obtained via the PCR-SBT method.
Table 1. Typing Results of HLA Class I Genes Using iGeneTech’s TargetSeq® Human HLA Panel V2.
Note 1: Expected results obtained via the PCR-SBT method (v3.30); Reported results captured by iGeneTech’s TargetSeq® Human HLA Panel V2 (v3.54).
Table 2. Typing Results of HLA Class II Genes Using iGeneTech’s TargetSeq® Human HLA Panel V2.

Note 2: Sample 6’s DRB115:01 and DRB115:02 differ in the second numerical segment, speculated to be caused by a version discrepancy in the database used.
04 Product Information
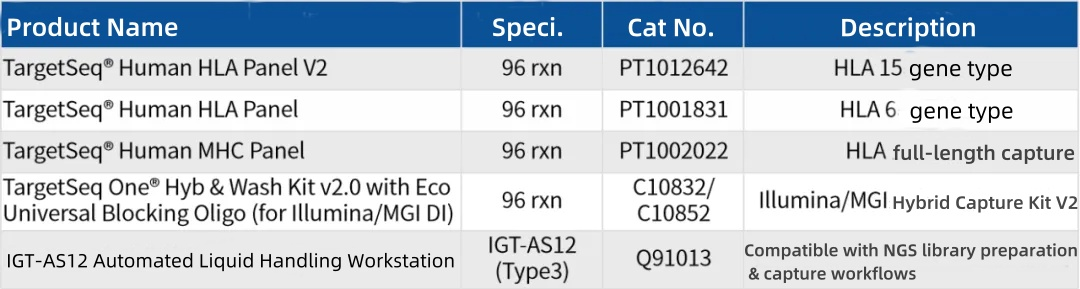

 CN
CN