Duchenne Muscular Dystrophy (DMD) and Becker Muscular Dystrophy (BMD) are X-linked recessive monogenic disorders caused by mutations in the DMD gene. They primarily affect males, who present with progressive muscle weakness, while female carriers may also exhibit mild symptoms.
The DMD gene is located at the Xp21.2 locus and is the largest known human gene, consisting of 2.22 million base pairs and containing 79 exons. It is responsible for encoding "dystrophin" --a protein that acts as a "structural scaffold" for muscle fibers, protecting muscles from damage during contraction. Most DMD patients present with deletion or duplication mutations. Multiplex Ligation-dependent Probe Amplification (MLPA) is the "gold standard" for detecting DMD deletions/duplications, but it cannot detect point mutations. If there are breakpoints or point mutations in the probe-binding region, it can lead to missed detections.

A team led by Dr. Tan from the Second Affiliated Hospital of Chongqing Medical University, in collaboration with the First Affiliated Hospital of Chongqing Medical University and other institutions, published a research finding titled A novel partial mRNA-derived duplication of the DMD gene identified in NGS carrier screening in the journal Journal of Genetics, which elucidated a rare DMD duplication fragment.
By adopting NGS carrier screening technology, the team successfully identified a novel partial mRNA-derived duplication variant of the DMD gene. This study is the first to identify such a new partial mRNA-derived duplication variant of the DMD gene, which is formed by the insertion of partially reverse-transcribed cDNA from a rare transcript into the non-coding region of chromosome 13. Through validation, this variant was classified as a benign variant.
Research Overview
The research team enrolled a 36-year-old pregnant woman at 7 weeks of gestation, who was detected with abnormal non-contiguous duplication variants in the DMD gene, involving exons 29–37 and exons 39–40. By adopting the technical workflow of "NGS screening–MLPA comparison–breakpoint analysis", the team resolved the detection contradictions of DMD duplication variants in clinical practice. This work not only identified a new type of benign variant but also clarified the core value of "breakpoint analysis" in DMD genetic diagnosis, providing important references for the detection and counseling of similar cases in the future.
iGeneTech was honored to provide custom probes and related kits for this research, including the IGT® Enzyme Plus Library Prep Kit V3 (library construction kit) and the TargetSeq One® Hyb & Wash Kit v2 (hybrid capture kit).
This case indicates that heterozygous duplication variants of the DMD gene detected in female carriers do not necessarily have pathogenicity, even if their range is large. During genetic counseling, a cautious assessment is required.

Original Article Link: https://doi.org/10.1007/s12041-025-01511-2
Product Advantages of iGeneTech
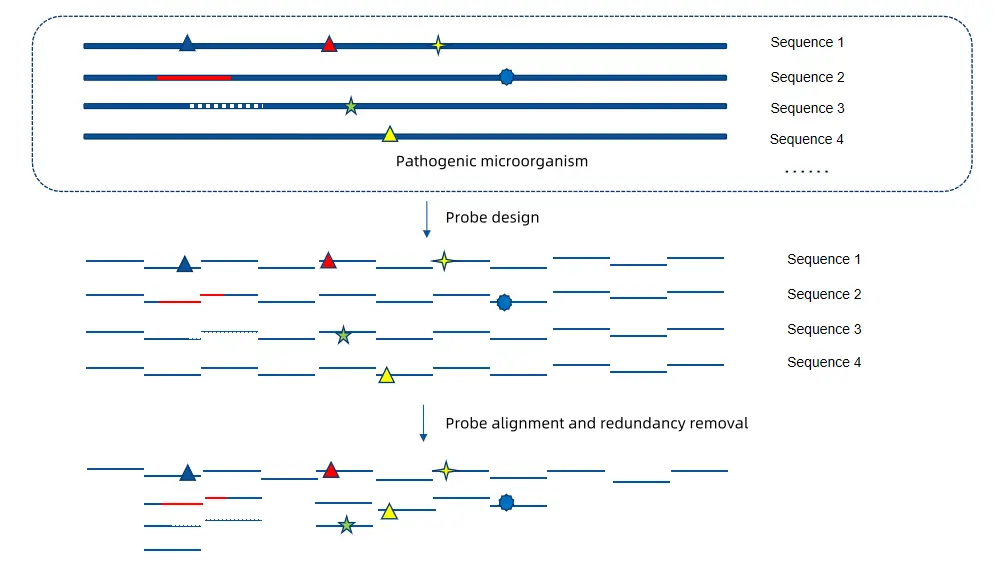
Relying on its independently developed TargetSeq® Hybrid Capture Sequencing Technology and IGT® Oligo Pools Large-Scale Parallel Synthesis Platform, iGeneTech can quickly provide various types of high-quality predefined and customized products. It offers an integrated solution covering the entire process from probe design, Oligo Pools synthesis, and probe preparation to the delivery of probes and capture kits.
Product Info


 CN
CN