In the field of cancer treatment, MRD (Minimal Residual Disease) testing has long been a major focus of attention. Like a "precision magnifying glass," it can capture tumor cells that are difficult to detect with conventional imaging tests, making it of great significance for patients' treatment and prognosis. However, this detection technology has fallen into a dilemma of being "highly useful for patients but challenging for doctors." Today, we will delve into the key issues and corresponding solutions.
Highly Beneficial for Patients
1. Predicting Recurrence Risk
Results from the DRIVE study (NCT06443684) showed that dynamic ctDNA monitoring can indicate disease progression a median of 8.7 months earlier than conventional imaging, with an advance of up to 11.3 months in the EGFR-mutant subgroup [1].
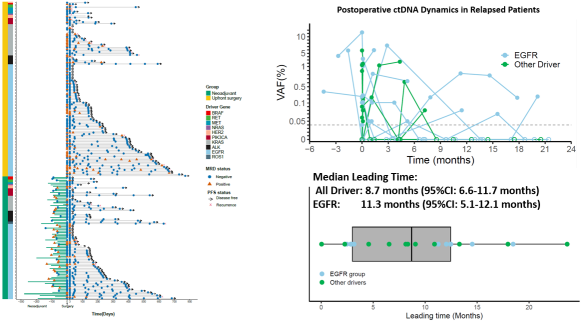
Figure 1 Dynamic ctDNA monitoring predicts patient recurrence prior to imaging [1]
2. Dynamically Assessing Treatment Efficacy
The DYNAMIC study published its 5-year results in Nature Medicine, validating that the ctDNA-guided adjuvant chemotherapy strategy significantly reduced the use of chemotherapy (15% vs. 28%) in patients with stage II colon cancer. Meanwhile, it maintained recurrence-free survival (RFS) and overall survival (OS) rates similar to those of conventional treatment (88% vs. 87% and 93.8% vs. 93.3%, respectively) during 5-year follow-up. Additionally, ctDNA clearance was associated with better prognosis, suggesting that dynamic changes in ctDNA monitored at multiple time points could be used to assess treatment efficacy and recurrence risk in the future [2].
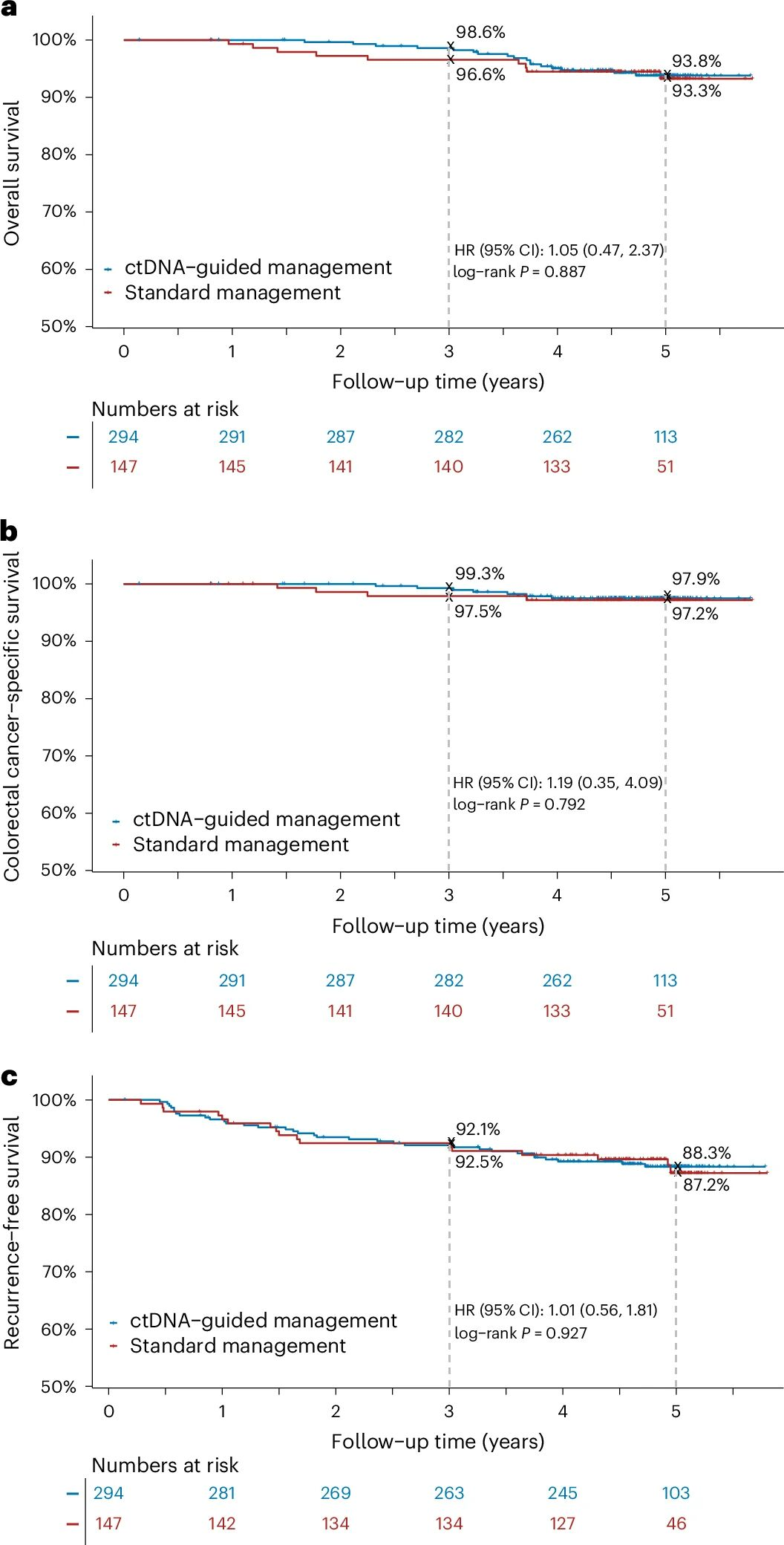
Figure 2 Summary of key survival data from 5-year follow-up of the DYNAMIC study [2]
Challenges in Clinical Adoption by Physicians
High Detection Cost Limits Clinical Application and Research Advancement
Currently, the cost of a single MRD test often exceeds several thousand yuan, making it unaffordable for many patients. Additionally, large-scale in-hospital testing requires supporting professional equipment and reagents, with upfront investment prohibitive for primary hospitals. This directly hinders research sample enrollment and slows the accumulation of evidence-based medicine.
Prognostic Value as the Main Focus; Clinical Evidence for Adjuvant Therapy Needs Improvement
Although domestic and international studies on ctDNA-guided adjuvant therapy have shown significant efficacy, such strategies have been established in only a few cancer types. Their value in guiding treatment decisions lacks validation from larger sample sizes and additional prospective, interventional studies.
IMvigor011 Study (Atezolizumab) [3]
ctDNA-positive patients: Adjuvant therapy with atezolizumab resulted in significant benefits, with median disease-free survival (DFS) (9.9 months vs. 4.8 months) and overall survival (OS) (32.8 months vs. 21.1 months) substantially prolonged compared to placebo.
ctDNA-negative patients: Recurrence risk was extremely low, with 1-year DFS rate of 95% and 2-year DFS rate of 88%, avoiding unnecessary treatment burden for low-risk (ctDNA-negative) patients.
CheckMate274 Study (Nivolumab) [4]
ctDNA-positive patients: Nivolumab significantly reduced disease risk, decreasing recurrence/death risk by 65% (DFS HR = 0.35, 95% CI 0.18–0.66) and death risk by 59% (OS HR = 0.41, 95% CI 0.20–0.83) compared to placebo, making this group the core beneficiaries of adjuvant immunotherapy.
ctDNA-negative patients: No significant difference in efficacy was observed between nivolumab and placebo (DFS HR = 0.99, 95% CI 0.51–1.93; OS HR = 0.87, 95% CI 0.41–1.84). Treatment de-escalation may be considered to reduce toxicity and medical expenditures.
Highly Complex Detection Workflow Hinders Hospital-Based Implementation
Compared to conventional PCR testing, MRD testing imposes extremely high requirements on hospital technical capabilities, equipment, and management systems:
Core operational steps such as library construction and hybridization capture are cumbersome, demanding rigorous professional competence of operators and high precision of equipment.
Additionally, cfDNA samples have low initial input quantities, and detection sensitivity requirements of 0.01% necessitate precise control throughout the entire experimental process.
Meanwhile, personalized custom probes require successful one-time synthesis, placing strict demands on probe design schemes and suppliers’ production/delivery efficiency.
Therefore, implementing MRD testing in hospitals requires establishing a professional technical team, improving process management systems, and equipping full sets of high-performance reagents and equipment. Deficiencies in any link will hinder the progress of testing.
Solutions to Overcome These Challenges
1. Free Synthesis of Personalized Custom Panels
Based on hundreds of loci identified through preliminary Whole-Exome Sequencing (WES) screening, we offer free probe synthesis and a complete reagent solution. While ensuring the accuracy of detection loci and results, this effectively reduces costs for both patients and research institutions, creating conditions for more patients to access testing and more clinical studies to be conducted.
Workflow Steps
WES Screening for Somatic Variants→Mutant Locus Selection→Free Customization of MRD Panel→NGS Dynamic Monitoring
Technical Advantages
l AIExome V5 Tumor Version: Panoramic scanning of genetic variation profiles, with differential depth enhancing detection sensitivity;
l End-to-end streamlined design, synthesis, and ordering system, with rapid delivery within 5 working days;
l UMI molecular tags facilitate efficient molecular recovery; Ultra-high depth (≥100,000×) enables stable monitoring of ultra-low-abundance ctDNA molecules.
2. Customization Turnaround Time Reduced to 3–5 Days
The PRECARE study advanced the blood collection time window for post-operative initial MRD testing to 7 days after surgery [5]. This requires that the entire workflow (including personalized probe design and customization) be completed within 3–5 days.
In response to this, iGeneTech has launched an online design platform that supports users in submitting designs at any time and performing one-click ordering. Leveraging mature algorithms, dedicated workflow routing, and real-time progress tracking, it enables users to efficiently obtain high-quality personalized custom Panels.
Subsequently, paired with end-to-end supporting reagents, stable MRD testing can be achieved.
3. Sample-to-Library Automated Solution
iGeneTech’s IGT-AS01 portable modular NGS workstation is an “on-demand testing” automated solution. It integrates the entire workflows of pre-library construction and probe hybridization capture into a dedicated reagent strip: single samples can be run independently with unattended operation, and physically isolated units eliminate cross-contamination.
With a footprint of only 0.1 m² and a height of 34 cm, the device can be placed in a biosafety cabinet, and multiple units can be matrix-expanded to increase throughput. By simplifying workflows and reducing manual dependency, it helps hospitals efficiently establish a hospital-based MRD testing platform.

iGeneTech’s free customization solutions, online design and workflow routing systems, complete reagent portfolios, and automated solutions are offering more options for the advancement of MRD clinical research and its hospital-based implementation. To truly promote the widespread clinical application of MRD testing in solid tumors and fully realize its value, conducting more clinical studies and accumulating more clinical evidence is crucial.
Numerous positive developments have been achieved both domestically and internationally. It is believed that with the accumulation of clinical evidence and wider adoption of technology, MRD testing will evolve from a "cutting-edge technology" to a "standard tool" in precision therapy for solid tumors. This will truly make the concept of "precision cancer prevention and on-demand treatment" a reality, benefiting more patients.
References
[1]. Prospective Observational Study on Dynamic Monitoring of Residual DrIVer Mutations for DiseasE Recurrence in Stage III NSCLC,DRIVE Study,NCT06443684
[2]. Tie J, Wang Y, Lo S N, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: 5-year outcomes of the randomized DYNAMIC trial[J]. Nature Medicine, 2025
[3]. Powles T, Kann AG, Castellano D, et al. ctDNA-guided adjuvant atezolizumab in muscle-invasive bladder cancer. N Engl J Med 2025;393:2395-408
[4]. Galsky MD, Gschwend JE, Milowsky MI, et al. Adjuvant nivolumab versus placebo for high-risk muscle-invasive urothelial carcinoma: 5-year efficacy and ctDNA results from CheckMate 274. Ann Oncol. Published online October 17, 2025. doi:10.1016/j.annonc.2025.09.139
[5]. Cao D, Lv GZ, Li C, et al. Association of Recurrence with a Tumor-informed Personalized ctDNA Detection Approach in Resectable Colorectal Cancer: Results of a Prospective Observational Study. Ann Surg. Published online November 3, 2025. doi:10.1097/SLA.0000000000006971
Product Info
Product Name | Speci. | Cat. No |
IGT® Fast Library Prep Kit v2.0 | 96 rxn | C10022 |
IGT® Adapter & 10 nt UDI Primer 1-768 (for Illumina, plate) | 768 rxn | C11282 |
Tumor-informed MRD Research Kit v2.0 (for Illumina) | 96 rxn | C11592 |
IGT-AS12 Automated Liquid Handling Workstation | Configuration III | Q91013 |
IGT® Enzyme One-Strip (ILM-UDI) | 96 rxn | C11692 |
IGT® Enzyme One-Strip (MGI-UDI) | 96 rxn | C11702 |
IGT® Fast One-Strip (ILM-UMI) | 96 rxn | C11752 |
IGT® Fast One-Strip (MGI-UMI) | 96 rxn | C11762 |

 CN
CN



