01 Proven and Effective Detection Solution
Currently, among domestic and international detection solutions, after multiple comparisons and validations of clinical data between the fixed-panel approach and the personalized approach, the personalized MRD custom Panel solution has mostly prevailed. However, this complex and personalized solution has posed unprecedented challenges to many clinical testing companies. The market is in need of a supplier that can provide stable, standardized, and efficient personalized custom Panels.
How are we addressing this? For the personalized MRD Panel customization process, we have developed an online design platform, which facilitates customers to perform Panel management, submit designs, place orders, and initiate synthesis at any time. The platform supports automatic workflow, dedicated-line production, and delivery—with no manual intervention required throughout the entire process. Through standardized process verification and fast dedicated-line workflow, we ensure the stable, accurate, and efficient production and delivery of personalized custom Panels within a standardized process.
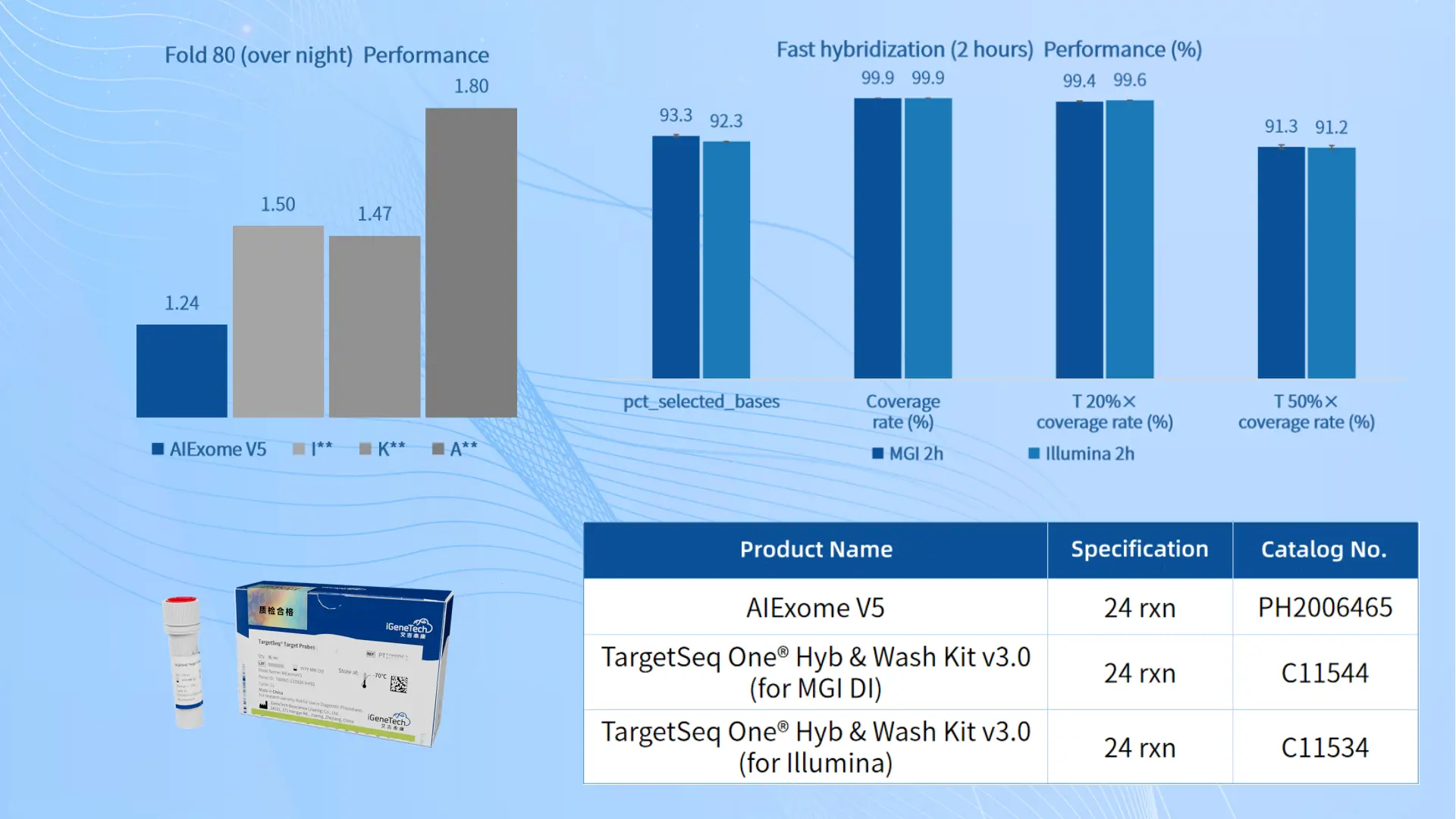
In terms of probe design, we have 11 years of experience and have developed a mature standardized design process, which has been successfully validated in over 3,000 custom Panels. This allows us to proactively identify and alert about high-risk regions when dealing with diverse design regions, and implement proactive interventions (such as incorporating mutant-specific probe designs and adjusting probe density) to ensure successful design in one go.
In the probe synthesis phase, our high-throughput DNA synthesis platform—Ignite 3.0—can synthesize Oligo Pools with a per-unit yield of over 0.5 fmol and a 95th/5th percentile ratio as low as 1.82, ensuring uniform sampling of probes across different regions. Meanwhile, the average single-base error rate (including mismatches, insertions, and deletions) is 0.1%, which is far lower than the industry average.

02 Practical and Accessible Detection Costs
For domestic cancer patients, one of the most significant current barriers to MRD detection is its high cost. Medical insurance coverage for MRD detection is still a distant prospect—each personalized MRD test costs 3,000–5,000 RMB, which is over 10 times more expensive than traditional tests used for prognostic monitoring, such as CT scans or tumor marker tests.
As we work toward securing medical insurance coverage, in addition to robust detection solutions, hospitals and clinical testing companies must address two key questions: how to make testing costs accessible to patients, and how to further reduce detection costs to sustain the development of MRD detection products. From a cost allocation perspective, the cost of universal reagents can be amortized across all NGS tests, while the cost of personalized customization is a fixed expense that a single patient must bear upfront. Even for panels with 10–30 sites, the customization cost alone is approximately 1,500 RMB. Given that MRD testing may not be continuous, it is generally necessary to include the customization cost in a single test. Therefore, the most critical step in cost reduction is to minimize customization fees—without reducing the number of monitoring sites, which is essential for ensuring the final test sensitivity.
So, how low is "low enough" for customization fees? Our solution is straightforward: free customization. This is not limited to 10–50 sites; instead, we offer free customization for up to 150 sites, which can reliably cover all sites identified in the initial screening. Even if the number of initially screened sites is small, designing multiple layers of probes within the 150-probe limit ensures that each site is firmly captured in subsequent tests. Furthermore, our bundled packages for universal reagents provide complete, efficient, and cost-effective hybrid capture reagent solutions. When paired with custom Panels (with no restrictions on the number of probes), these packages deliver high-quality hybrid capture data, thereby multiple safeguarding the sensitivity and accuracy of MRD detection.
03 Stable In-Hospital Process Implementation
With the improvement of the regulatory framework, there is an increasingly practical and urgent need for hospitals to implement localized MRD testing.
How can we ensure the stable implementation of the entire testing process in various hospitals? This requires consideration of supporting hardware, software, and the technical capabilities of laboratory staff.
A single server can support both the analysis system and interpretation system software—only regular online database updates and maintenance are needed. However, sample library preparation, hybrid capture experiments, and the associated equipment pose greater challenges for in-hospital implementation. In recent years, automated equipment has emerged rapidly; automated nucleic acid extraction, library preparation, and capture are key steps to simplify staff operations and ensure the stability of test results.
To address this, we have developed a new automated device: the IGT-AS01. This is a portable, modular NGS workstation designed for "on-demand testing." When used with dedicated pre-packaged reagent strips, it not only enables rapid, independent processing of individual samples but also allows flexible throughput expansion via a multi-unit matrix. This fully meets the dual needs of laboratories: operational flexibility and an extremely fast reporting cycle.

Specifically, it has the following features:
High Integration: It integrates all processes of NGS pre-library preparation and probe hybrid capture into a single reagent strip, completely eliminating the need for "wet experiments" from now on.
Unattended Operation: A single sample can be run without the need to batch samples, realizing true "sample in, captured library out" and enabling on-demand testing of samples.
Contamination Prevention: Physically isolated single reaction units fundamentally eliminate cross-contamination at the source.
Multi-Unit Matrix: One computer can control multiple reaction units, and these units can form a matrix to meet experimental throughput requirements.
Compact and Independent: The device occupies only 0.1 square meters, allowing flexible deployment. With a height of just 34 cm, it can be directly placed in a biosafety cabinet to meet the demands of stringent clinical experimental environments. Each reaction unit in the matrix operates independently to ensure the continuous operation of laboratory testing.
As a service provider specializing in the custom development of gene capture products, supply of targeted sequencing laboratory solutions, NGS rapid testing services, and large-scale DNA synthesis, we have provided nearly 1,000 customers in China with hundreds of pre-fabricated gene capture products, high-performance reagent components, nearly 2,000 patient-specific customized MRD Panels, and over 4,000 high-standard personalized gene capture products.
We iGeneTech believe that the development of MRD products does not require handling all processes independently; instead, we can reach our common goals through faster and better industrial chain collaboration.

 CN
CN