As an emerging technology in the field of tumor monitoring, the detection of Minimal Residual Disease (MRD) has increasingly become one of the important indicators for evaluating the effectiveness of tumor treatment, prognosis assessment, and recurrence prediction. In this context, we will share the current research status of MRD detection from aspects such as the publication status of MRD-related articles in recent years, applicable cancer types, major events of ctDNA-MRD at home and abroad, as well as ongoing MRD research on targeted MRD treatment. With the maturation of MRD detection technology and the release of more clinical verification data, it is bound to lead to the promotion of its clinical application. It is believed that MRD detection will play an even more important role in future tumor treatment, benefiting more clinical patients.
Publication status of MRD-related articles and applicable cancer types
In recent years, the number of research papers published on MRD detection has remained high, and the range of applicable cancer types has become increasingly extensive. Initially focused on hematological tumors such as myeloma and acute lymphoblastic leukemia, in recent years, the application of MRD detection has gradually expanded to solid tumors including lung cancer, colorectal cancer, gastric cancer, and breast cancer [1-4]. The scope of application of MRD detection continues to expand. ctDNA-MRD detection has also demonstrated significant advantages as a prognostic assessment tool in the field of solid tumors. It not only helps to more accurately evaluate patients' prognosis but also provides a scientific basis for the formulation of personalized treatment plans. In particular, MRD detection plays an important role in post-operative or post-treatment management, patient selection for immunotherapy, and the choice of treatment methods and treatment breaks.
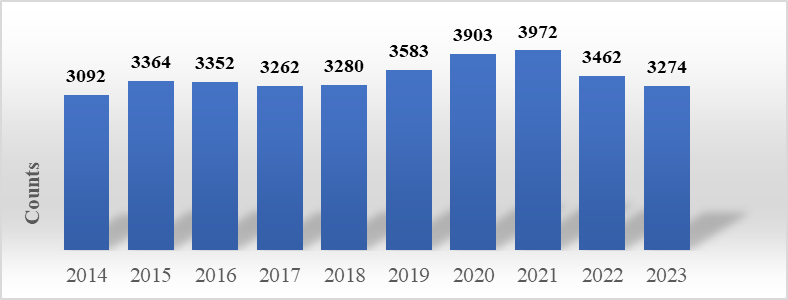
Figure 1. Number of MRD-related literatures retrieved from PubMed from 2014 to 2023.
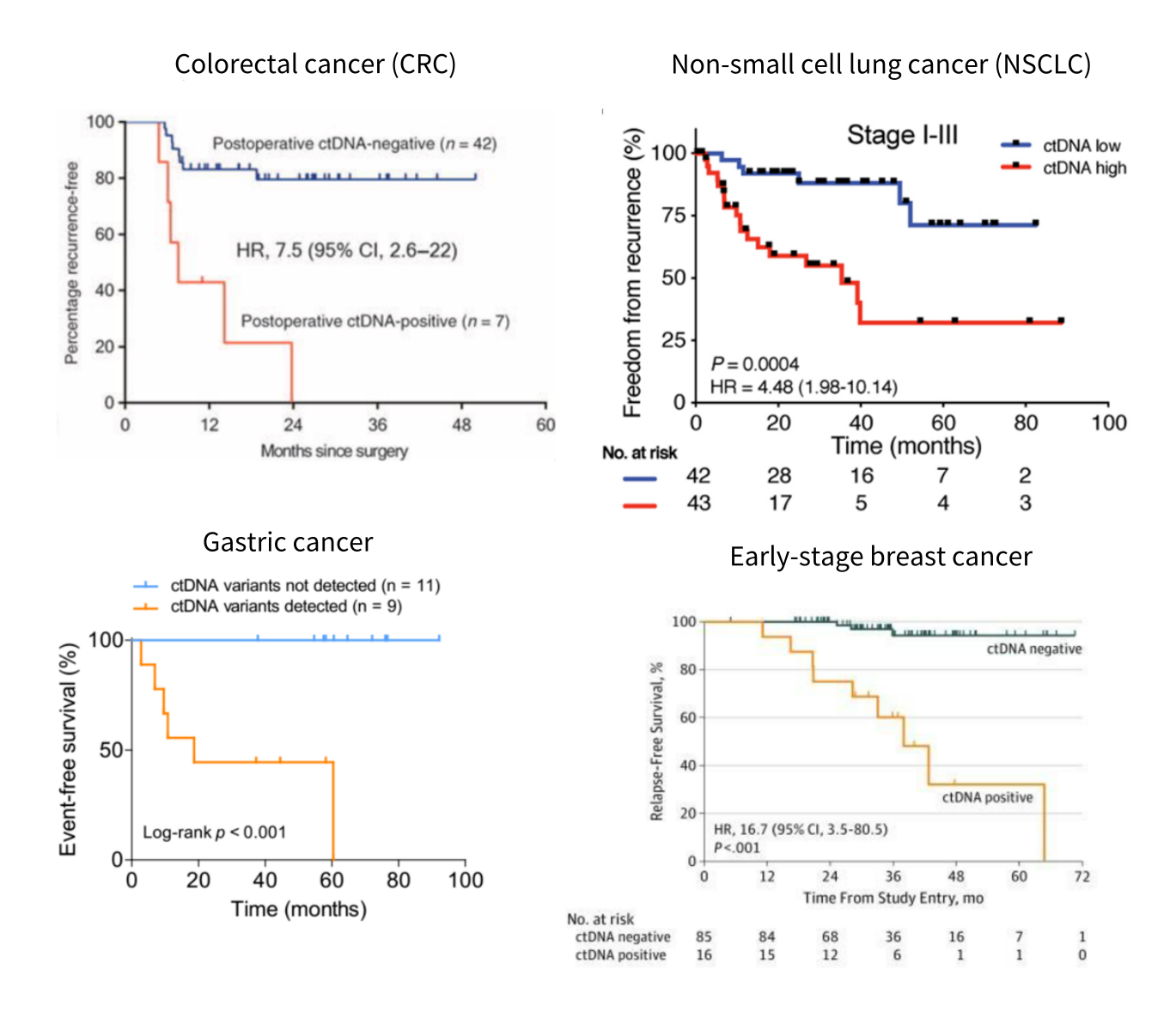
Figure 2. Post-treatment monitoring of ctDNA-MRD and disease progression in different cancer types
Key Developments of MRD Detection Globally and in China
Currently, MRD detection abroad for recurrence monitoring in multiple cancer types has been included in medical insurance coverage. Over the past five years, domestically, clinical practices in China have gradually explored the methodology and applicability of MRD in solid tumors. In 2021, following the release of China’s first Expert Consensus on Molecular Residual Disease (MRD) in Non-Small Cell Lung Cancer, MRD-related consensus documents and guidelines for various solid tumor types have been continuously updated. Recently, at the 13th Chinese Congress of Pathology in Beijing, Professor Wu Huanwen from Peking Union Medical College Hospital delivered a special academic report titled Consensus on Minimal Residual Disease (MRD) Detection in Solid Tumors (hereinafter "the Consensus"). The report addressed key topics including the definition of solid tumor MRD, its clinical value, target populations, technical approaches, strategies, and timing of detection, culminating in a formal consensus. The launch of this consensus represents a landmark achievement for MRD detection in solid tumors.
Over the past three years, nearly 20 MRD detection products have sprung up like mushrooms after rain in China. Alongside the release of more clinical research results, updates to consensus statements and guidelines, growing evidence-based medical support, and the expansion of MRD’s clinical applications through academic exchanges, MRD detection is poised to rapidly become a standard clinical practice, benefiting more patients.
Founded in 2014, iGeneTech is China’s first company to specialize in targeted capture solutions, leveraging three core proprietary technology platforms: NGS probe hybridization, multiplex PCR, and high-throughput Oligo Pools synthesis. Over the past decade, it has independently developed supporting reagent solutions, particularly driving innovations in MRD detection technologies. By collaborating with partners to enhance performance, reduce costs, and create flexible technical protocols, iGeneTech has provided comprehensive support for advancing the clinical adoption of MRD testing.
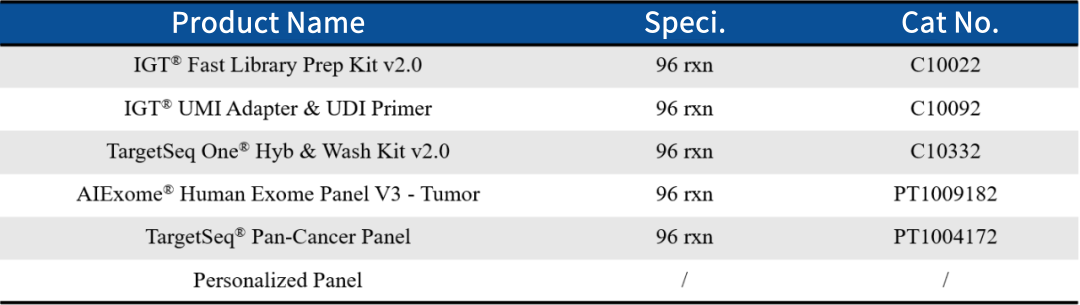

 CN
CN



