In March 2024, iGeneTech® officially launched its brand-new methylation-based multi-cancer early detection product, the BisCap® Multi-Cancer Early Detection Panel (MCED Panel), after multiple rounds of testing and performance validation. The MCED Panel targets a 27.7 kb region and covers 8 common cancer types: colorectal cancer, lung cancer, gastric cancer, esophageal cancer, liver cancer, prostate cancer, pancreatic cancer, and ovarian cancer, including 2,422 CpG sites. Paired with iGeneTech's self-developed single-stranded library preparation kit, this product efficiently captures trace methylation signals in blood, providing clinical support for multi-cancer early detection and screening.
Background Introduction
With the extension of human average lifespan, the threat of cancer has become increasingly prominent. Cancer has become the leading cause of death among urban and rural residents in China. Early detection of cancer remains the most effective method to reduce cancer mortality, which includes two components: early diagnosis and early screening.
Peripheral blood cell-free DNA (cfDNA) contains DNA fragments shed from tumor cells or in-situ tumor cells (circulating tumor DNA, ctDNA), whose carried information from primary tumor cells enables non-invasive liquid biopsy. In early-stage cancer patients, the ctDNA content in blood is extremely low. Most products currently approved by NMPA (National Medical Products Administration) are single-cancer methylation early screening products, primarily using PCR fluorescence probe technology. This method can only perform qualitative detection of a small number of methylation sites and is mainly targeted at high-risk populations for a specific tumor. For tumor early screening in sub-healthy and healthy populations, multi-cancer methylation detection kits offer higher cost-effectiveness.
iGeneTech developed the MCED Panel product by referencing tumor marker sites in cell-free DNA from blood-based population cohort studies published in numerous literatures, and leveraging its years of experience and advantages in probe design. This product can detect signals from 8 types of cancers in one test, assisting clinicians in non-invasive early screening of suspected tumor patients.
Product Features
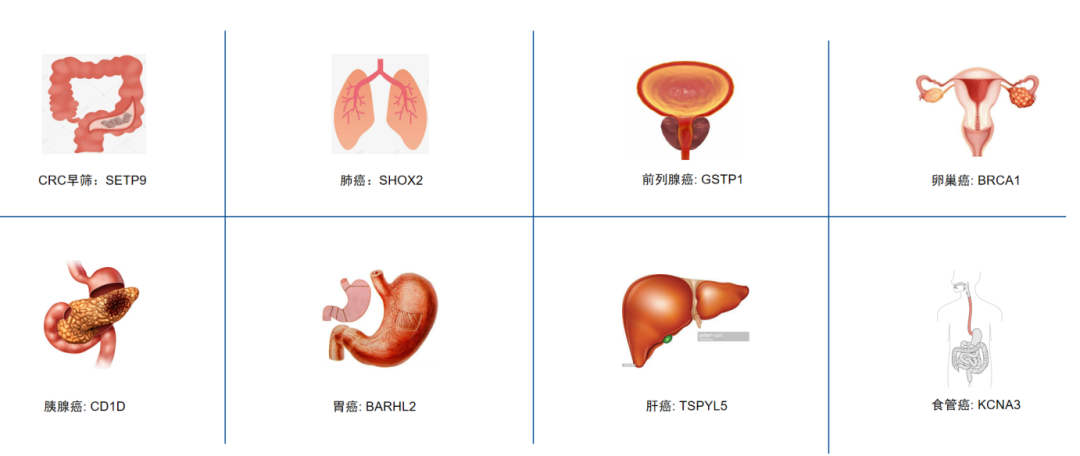
Against the backdrop of China's cancer landscape, we have selected 8 high-incidence cancer types: colorectal cancer, lung cancer, gastric cancer, esophageal cancer, liver cancer, prostate cancer, pancreatic cancer, and ovarian cancer. Drawing on CpG islands validated in FDA (U.S. Food and Drug Administration) and NMPA (National Medical Products Administration)-approved products, as well as key sites documented in cancer early screening literature, iGeneTech employs a bis-state design approach to design probes for both the positive and negative strands of CpG islands.
The self-developed single-stranded library preparation kit significantly enhances the utilization rate of original ctDNA molecules, enabling library construction with as low as 1 ng of cfDNA. When paired with the BisCap® hybridization system, it sensitively captures faint cancer methylation signals.
During preclinical development, the product incorporated real-world tissue samples, paired leukocyte samples, and plasma cell-free DNA samples across all targeted cancer types. Advanced genomics and bioinformatics methodologies were utilized for data analysis and validation. Additionally, self-developed single-cancer analysis models and threshold models were established to improve the accuracy and efficiency of early tumor diagnosis, providing robust support for methylation-based multi-cancer early screening.
Product Advantages
Superior Performance: The capture workflow is highly optimized to enhance capture efficiency and stability, enabling efficient capture of multiple genetic loci.
Suitable for Low-Input Samples: Only 1 tube of blood (with as low as 1 ng of cfDNA) is required. Combined with iGeneTech’s self-developed single-stranded library preparation kit and BisCap® methylation hybridization capture kit, methylation detection can be performed.
Advanced Analytical Models: Intelligently identifies cancer signals and provides Methylation Score (MS) values for each cancer type, effectively reducing omissions and enabling early detection and precise localization of tumors.
NGS Data Performance
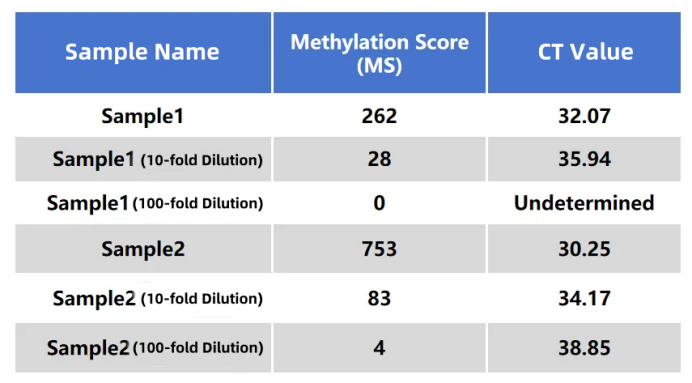
Figure 1. The MCED Panel paired with the BisCap® Hybridization System showed excellent performance across different sample types.
Samples included cfDNA, tumor tissue, leukocytes, and NA12878, with 8-10 ng input for library construction. Libraries were prepared using the IGT® ssDNA Library Prep Kit and IGT® ssDNA Adapter & UDI Primer (for Illumina). A total of 2 μg of pooled libraries (500 ng per library) were used for four hybridizations, captured with the MCED Panel, and sequenced on the NovaSeq 6000 platform with PE150 chemistry, generating approximately 0.5 Gb of sequencing data.
Performance verification of tumor early screening in real-world samples
01 Colorectal Cancer
Test Results of Colorectal Cancer Tumor Tissue Samples
The results of the MCED Panel were highly consistent with those of a certain IVD colorectal cancer early screening kit (fluorescence quantitative PCR method) (R²=0.97).
Table 1. Data performance of tumor samples in the MCED Panel and the IVD colorectal cancer kit (fluorescence quantitative PCR method). Two clinical tumor samples were diluted 10-fold and 100-fold, respectively, and the same batch of samples were tested using the MCED Panel and the IVD colorectal cancer kit (fluorescence quantitative PCR method).
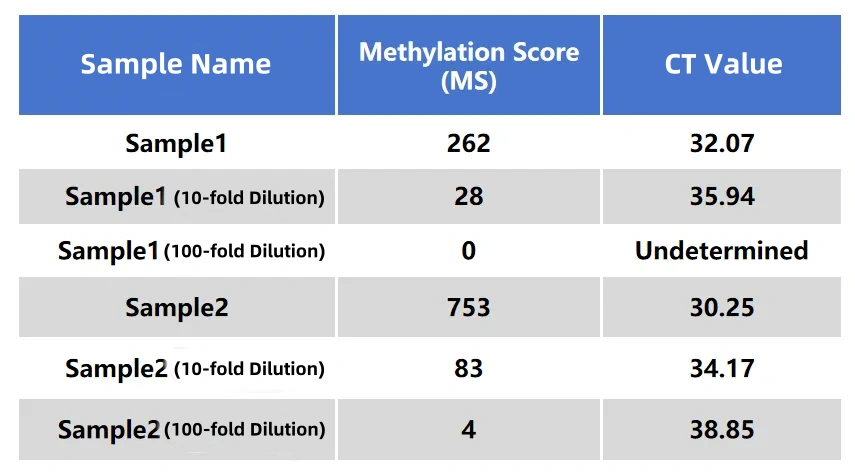
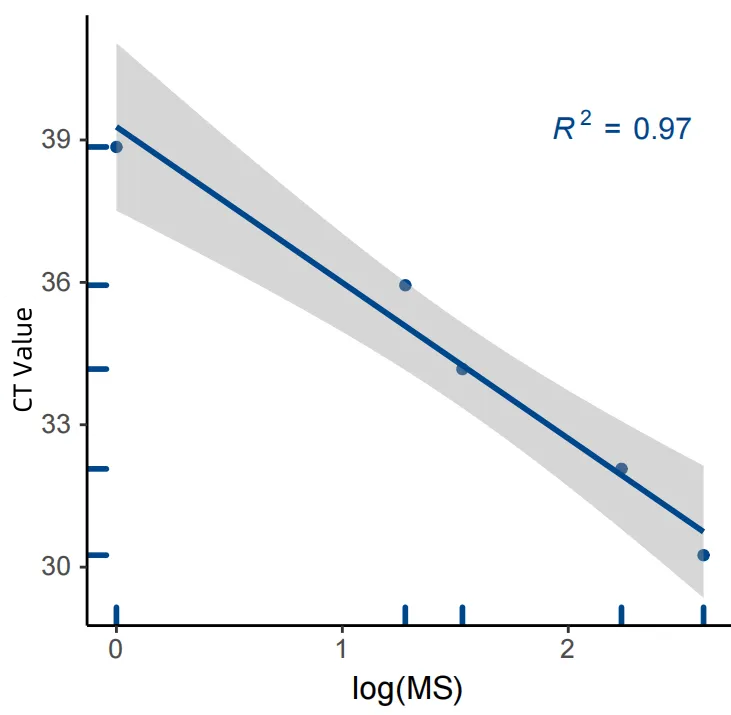
Figure 2. Consistency results between the MCED Panel and the colorectal cancer verification kit (fluorescence quantitative PCR method).
Test Results of Colorectal Cancer cfDNA Samples
The regional methylation difference indices of the corresponding cancer types in the MCED panel were analyzed for 3 clinical colorectal cancer tissue samples, 10 clinically determined colorectal cancer ctDNA samples, and 33 normal human cfDNA samples. According to iGeneTech's self-developed algorithm, tumor tissues, tumor ctDNA, and cfDNA from healthy populations in the real world can show significant differences based on the iGeneTech MS value standard.
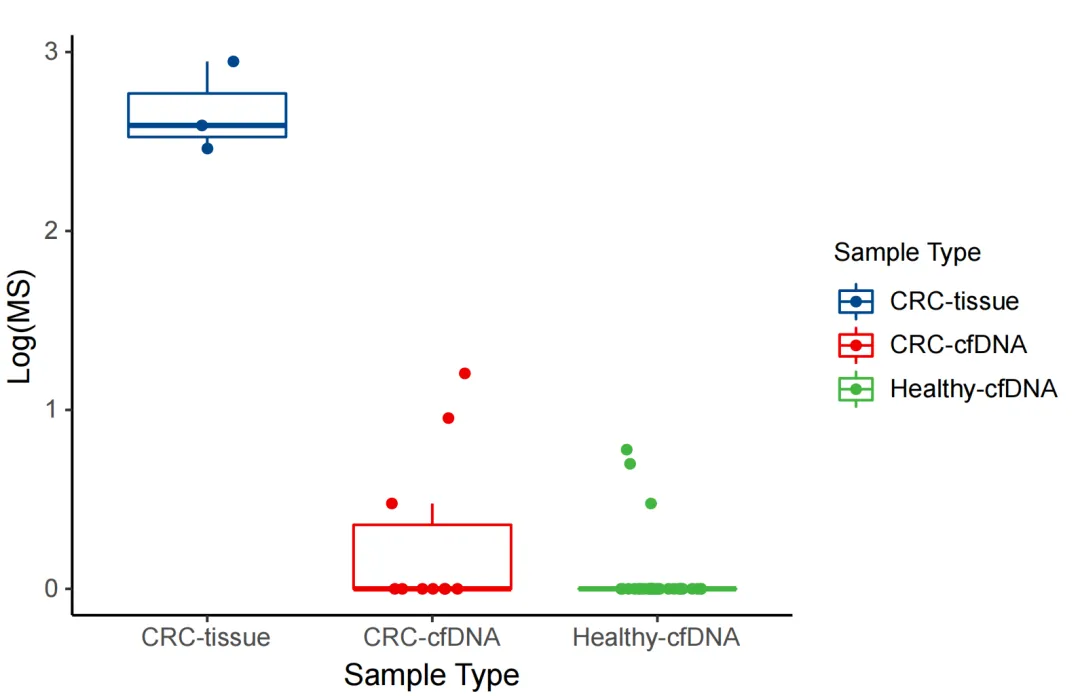
Figure 3. MS values of the MCED Panel in colorectal cancer patients and normal human samples.
02 Lung Cancer
Test Results of Lung Cancer cfDNA Samples
The regional methylation difference indices of the corresponding cancer types in the MCED panel were analyzed for 3 clinical lung cancer tissue samples, 5 clinically determined lung cancer ctDNA samples, and 33 normal human cfDNA samples. According to iGeneTech's self-developed algorithm, tumor tissues, tumor ctDNA, and cfDNA from healthy populations in the real world can show significant differences based on the iGeneTech MS value standard.
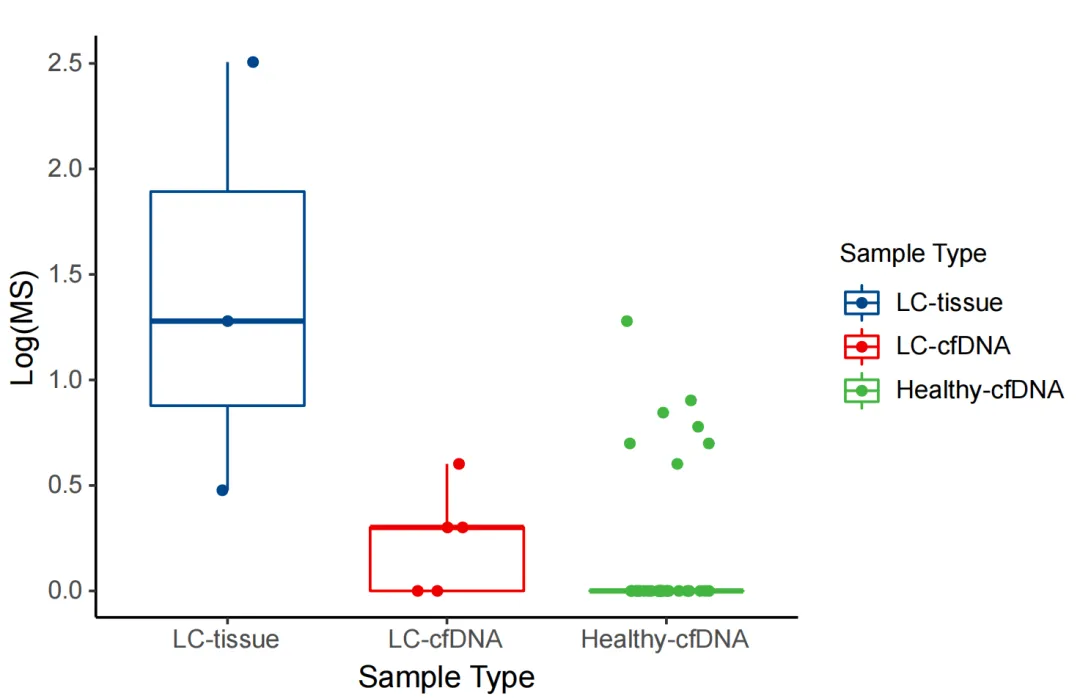
Figure 4. MS values of the MCED Panel in lung cancer patients and normal human samples.
03 Gastric Cancer
Test Results of Gastric Cancer cfDNA Samples
The regional methylation difference indices of the corresponding cancer types in the MCED panel were analyzed for 13 clinical gastric cancer tissue samples, 5 clinically determined gastric cancer ctDNA samples, and 33 normal human cfDNA samples. According to the self-developed algorithm of iGeneTech, tumor tissues, tumor ctDNA, and cfDNA from healthy populations in the real world can be clearly differentiated using the iGeneTech MS value as a criterion.
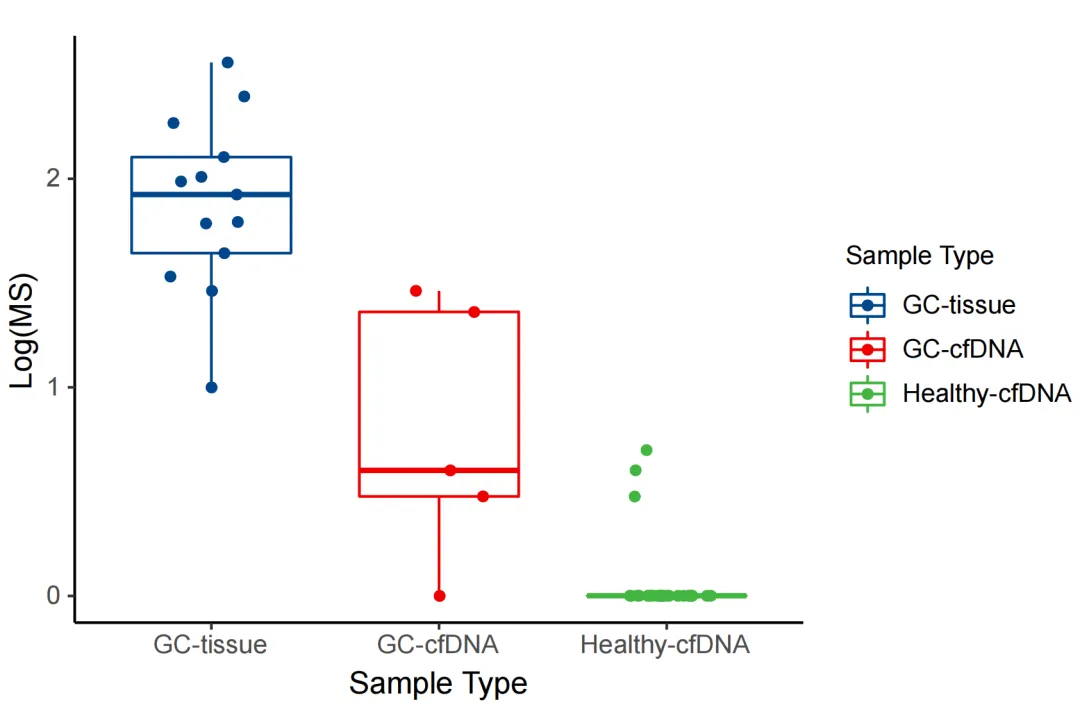
Figure 5. MS values of the MCED Panel in gastric cancer patients and normal human samples.
04 Esophageal Cancer, Prostate Cancer, Liver Cancer, and Pancreatic Cancer
Test Results of Paired Tumor Samples for Esophageal Cancer, Prostate Cancer, Liver Cancer, and Pancreatic Cancer
Regional methylation difference indices of the corresponding cancer types in the MCED panel were analyzed for 4 pairs of tumor control samples and 33 normal human cfDNA samples. According to iGeneTech’s self-developed algorithm, tumor tissues, white blood cells, and cfDNA from healthy populations in the real world can be clearly differentiated using the iGeneTech MS value as a criterion.
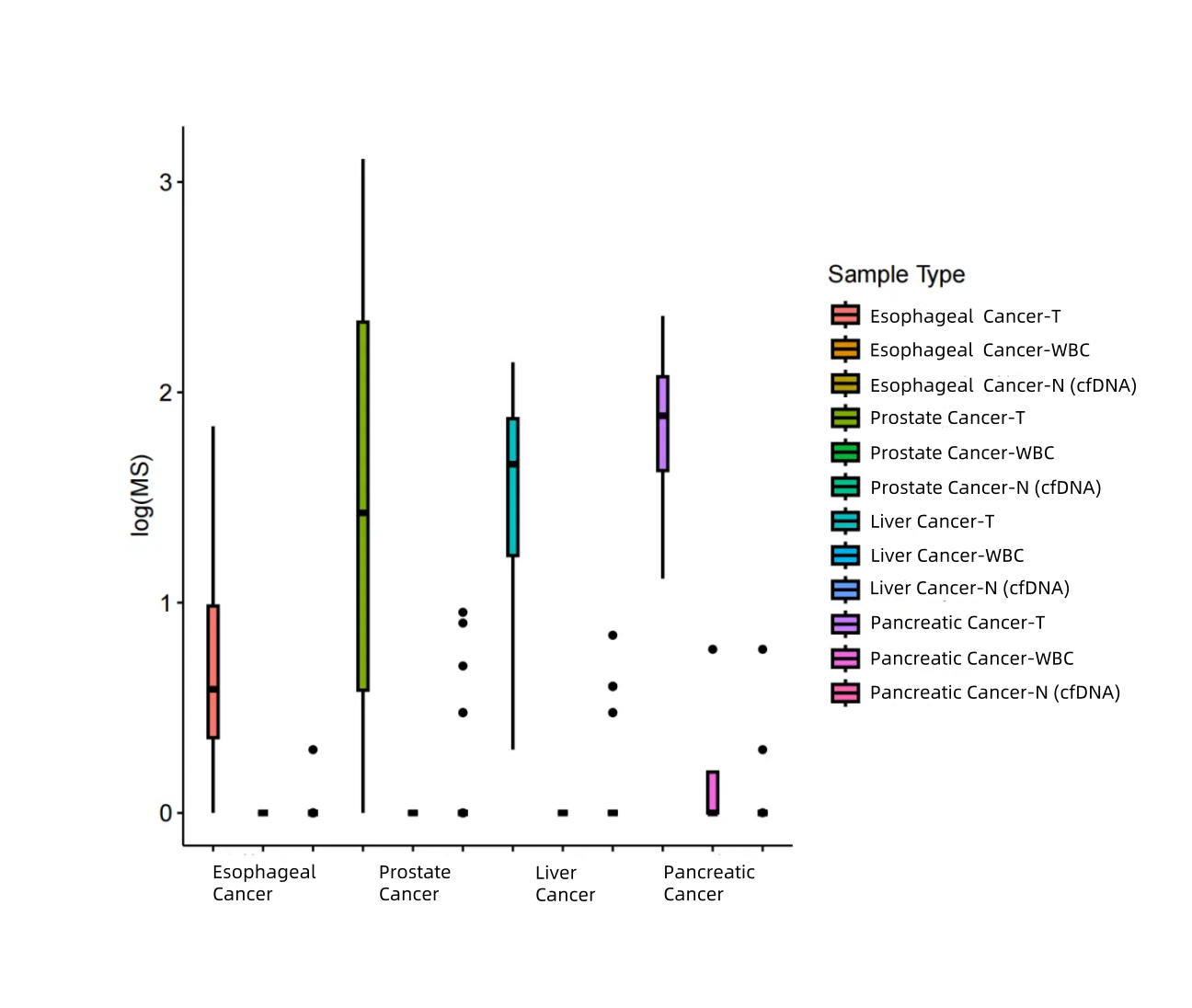
Figure 6. MS values of the MCED Panel in patients with esophageal cancer, prostate cancer, liver cancer, pancreatic cancer and normal human samples.
Conclusion
DNA methylation modification is an epigenetic phenomenon. Changes in DNA methylation can occur early in cancer development and are associated with numerous abnormal alterations in cancer cells. Therefore, DNA methylation analysis can effectively identify clinically significant tumor methylation markers. iGeneTech's self-developed whole-genome CpG island predefined product, the CpG Island Panel, is suitable for screening tumor methylation markers across the entire genome. The screened markers can be customized into tumor early screening Panels. Technically, the MCED Panel developed by iGeneTech, combined with the methylation single-strand library construction kit and BisCap® hybridization system, enables simultaneous detection of ctDNA methylation signals across multiple cancer types.
It should be noted that due to the nature of research and clinical trials, the advancement of tumor early screening is an ongoing process without a fixed endpoint. Therefore, for the latest research progress and results, iGeneTech can customize exclusive methylation detection products for clients based on diverse testing needs, including integrated services such as probe design, Panel performance testing, and analysis process development. Meanwhile, iGeneTech provides a full set of methylation capture reagents and automated experimental equipment, compatible with NGS sequencing platforms such as Illumina, GenomiCare, and MGI, to facilitate the rapid implementation of clients' methylation detection services.
Order Information
References
Sung H, Ferlay J, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4. PMID: 33538338.

 CN
CN