PARP inhibitors are medical agents that inhibit DNA damage repair in tumor cells, promote apoptosis of tumor cells, and thereby enhance the efficacy of radiotherapy as well as chemotherapy with alkylating agents and platinum-based drugs. Utilizing the unique mechanism of "synthetic lethality" against tumor cells [1], they have opened up new avenues for cancer treatment and have been widely applied in clinical practice.
At present, the U.S. FDA and China NMPA have approved a variety of PARP inhibitors for clinical use, with a wide range of approved indications covering ovarian cancer, fallopian tube cancer, primary peritoneal cancer, etc. [2]. In China, several PARP inhibitors such as Olaparib and Niraparib have also been successively approved for corresponding indications, and some have been included in medical insurance, fully demonstrating their important position in clinical treatment.
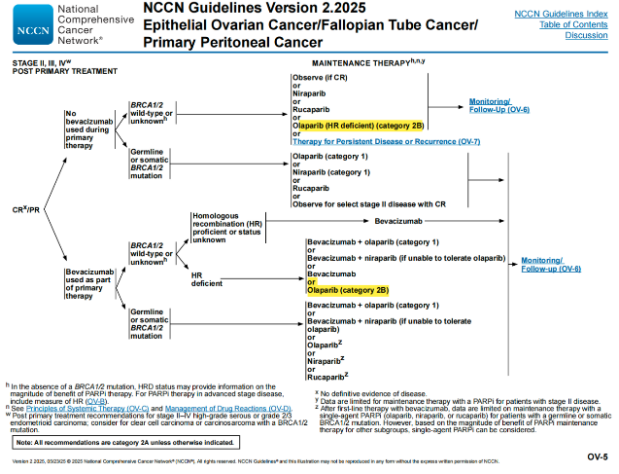
In the updated content of the NCCN Ovarian Cancer Guidelines (2025 Version 2), Olaparib single-agent has been newly added as a Category 2B recommendation for first-line maintenance therapy in patients with BRCA1/2 wild-type or unknown-type and HRD-positive status [3]. This update further highlights the significant clinical value of HRD status in precision treatment decision-making and broadens the applicable population for PARP inhibitors.
In addition to the relevant clinical research and progress in the updated guidelines, the final overall survival (OS) data of the L-MOCA study were officially released at this year's ESMO Annual Meeting [4]. The median OS of HRD-positive BRCA wild-type patients reached 54.6 months, which was higher than the median OS of 51 months in the overall population, demonstrating that HRD-positive BRCA wild-type patients can also achieve favorable survival benefits from olaparib maintenance therapy.
HRD test is crucial for accurately identifying patients who can benefit from PARP inhibitor therapy. With the continuous expansion of the clinical application of PARP inhibitors, accurate detection of HRD status has become a key link in meeting clinical treatment needs and realizing precision medicine.
iGeneTech HRD-related Products
iGeneTech has launched HRD testing-related products since 2020 and has continuously innovated in the application of HRD testing. It has not only achieved an efficient deep difference scheme for co-detection of HRD & HRR, but also customized multiple co-detection products containing HRD according to needs based on a flexible probe panel mixing scheme.
With changes and expansion of market demand, it can also quickly respond to the streamlining of the number of loci and locus screening for different populations, and develop high-quality HRD testing products.
Different numbers of loci ensure the uniform distribution of chromosomes
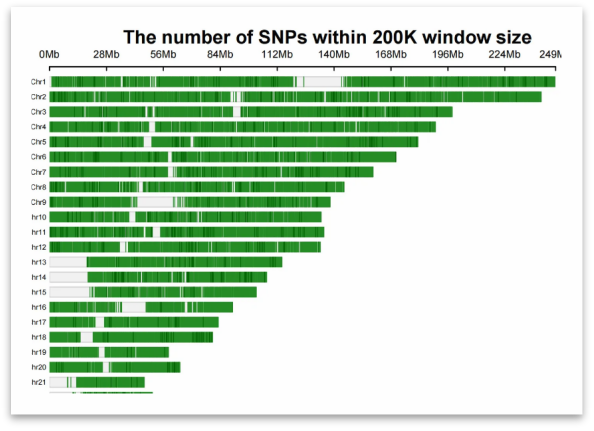
⬆: 12,128 Loci Probes
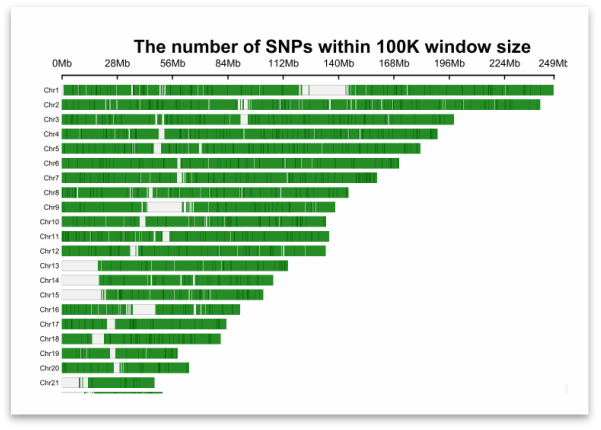
⬆: 21,239 Loci Probes
Figure. Analysis Density Map of Autosomes with Different Numbers of Loci
Product Performance Demonstration
1. Demonstration of Probe Capture Performance
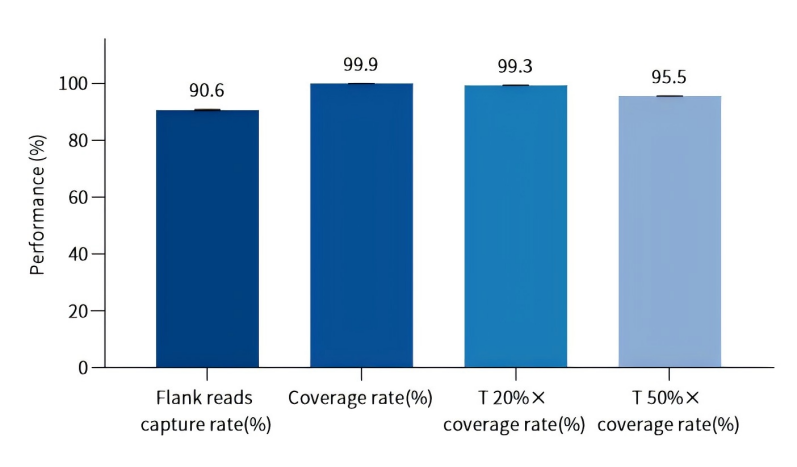
Figure. Testing capture performance with 21,239 loci probes
2. Detection of HRD Reference Standards
Table 1. Scoring results of different loci probes for various HRD reference standards
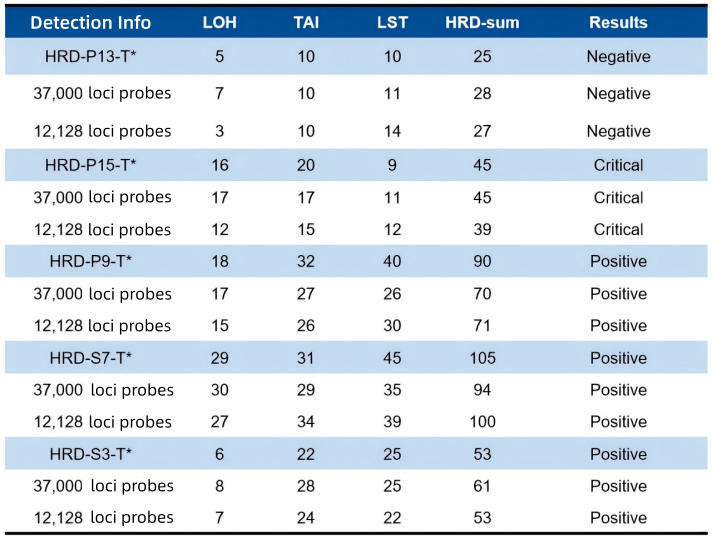
Note: HRD quality control products from Shuimu Jiheng (HRD-S7; HRD-S3) and Kebai (HRD-P13; HRD-P15; HRD-P9) were tested and scored using 37,000 loci probes and 12,128 loci probes.
Product Info
Product Name | Speci. | Cat. No |
HRD Panel | 24/96 rxn | PH2000975/PH2000972 |
HRR Panel | 24/96 rxn | PH2003715/PH2003712 |
HRD&HRR Panel | 24/96 rxn | PH2003805/PH2003802 |
AIExome® Human Exome Panel V5-Tumor | 24/96 rxn | PH2007385/PH2007382 |

 CN
CN