In September 2014, iGeneTech was quietly founded in Zhongguancun Life Science Park, Beijing. At that time, the core technologies in the upstream of the genetic testing industry were monopolized by foreign countries, leaving domestic enterprises facing a "bottleneck" predicament.
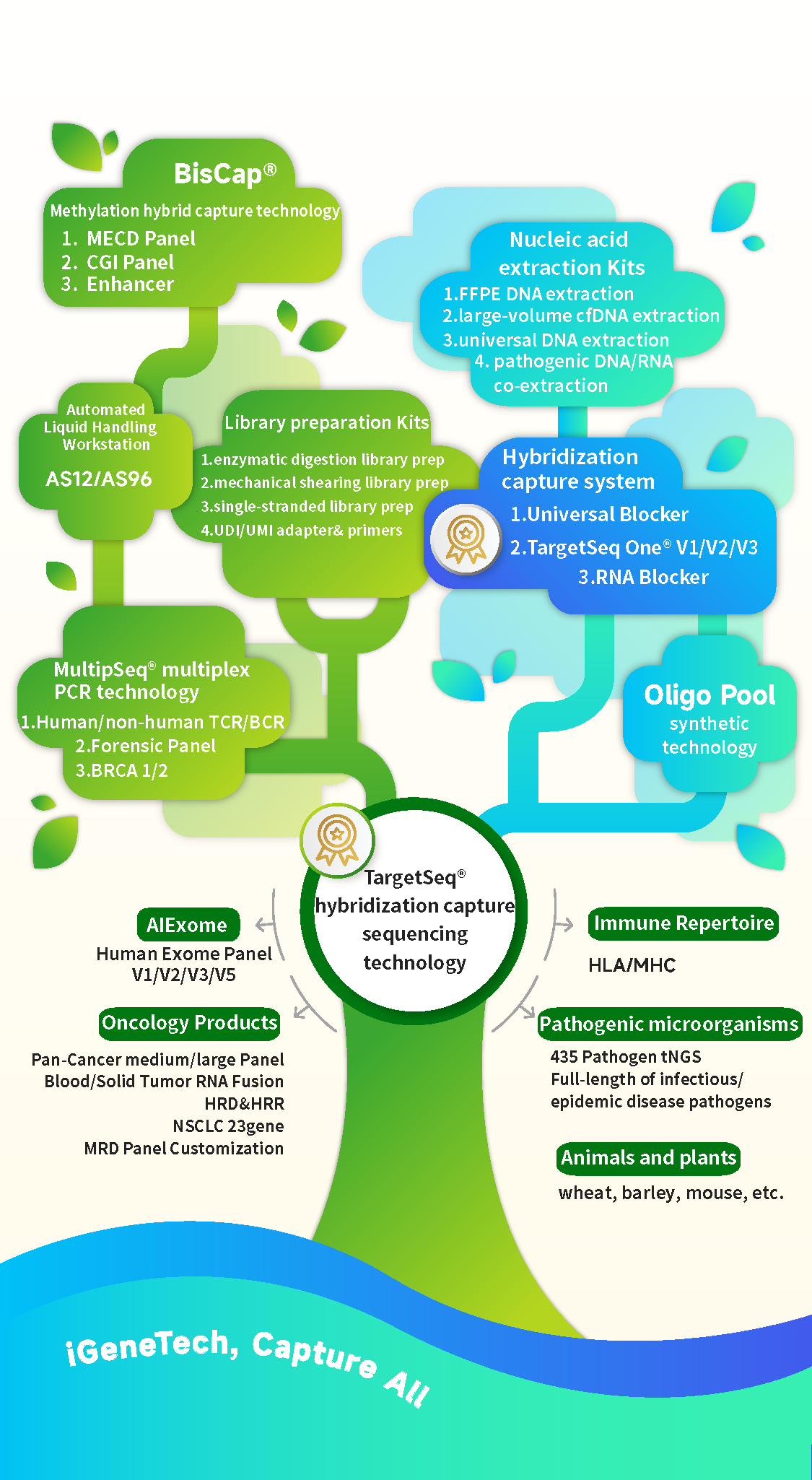
Ten years later, today, iGeneTech is committed to providing customized development of gene capture products and complete targeted sequencing solutions for multiple fields such as oncology, genetics, immunology, pathogenic microorganisms, agriculture, forensics, ancient DNA detection, and general health. This is achieved through independently developed technologies including Oligo Pool large-scale parallel synthesis technology, probe & primer design technology, hybrid capture sequencing technology TargetSeq®, multiplex amplicon sequencing technology MultipSeq®, methylation capture sequencing technology BisCap®, combined with a series of self-developed automated equipment (IGT-AS01, IGT-AS12, IGT-AS96).
Over the past decade, it has customized nearly 5,000 types of kits for customers, produced more than 11 million test kits (in terms of reaction counts), and accumulated experience in extracting over 500,000 samples and capturing and sequencing over 1,000,000 samples.
Team Building
Over a decade of leadership, the company's core technical team is led by more than ten doctors specializing in genetics and tumor molecular biology, as well as experts in primer and probe design, all with research experience in targeted capture sequencing technology. The core operational team comprises professionals with years of mature enterprise management experience. The R&D team consists of talents in related fields such as nucleic acid chemistry, reagent development, bioinformatics R&D, and instrument R&D. With sensitive and forward-looking strategic vision, they continuously drive product iteration and technological innovation at iGeneTech.
Quality System
With ten years of dedication, iGeneTech has obtained multiple compliance production quality management system certifications including ISO 13485 and ISO 9001, relying on stable and reliable production service processes. It has also independently developed and established a fast and traceable online quality management system, adhering to stringent quality control standards covering all-round factors such as raw materials, production processes, process control, and storage conditions. This ensures that each batch of products meets the expected standards and that different batches can provide reliable and consistent results in practical applications.


Honors and Qualifications
With a decade of dedicated efforts, iGeneTech has been focusing on gene capture, continuously engaging in R&D and innovation, and consolidating core technologies. It has been recognized as a National High-Tech Enterprise, Beijing "Specialized, Refined, Characteristic, and Innovative" Small and Medium-sized Enterprise, Zhejiang Provincial High-Tech Enterprise, Zhongguancun High-Tech Enterprise, Beijing Intellectual Property Pilot Unit, etc. Meanwhile, it has also won multiple awards in entrepreneurship competitions.


iGeneTech participated in the formulation of national standards including GB/T 37872-2019 General Rules for Quality Evaluation of Target Gene Region Capture and GB/T 44322-2024 Basic Requirements for Quality of Forensic Science Next-Generation Sequencing Reagents, as well as the compilation of the Expert Consensus on Clinical Detection and Application of Homologous Recombination Repair Deficiency (2021 Edition).
Its oncology products participated in the analytical performance evaluation and validation project based on next-generation sequencing technology (SEQC2) globally conducted by the MicroArray/Sequencing Quality Control (MAQC) Consortium led by the US FDA, and are at a leading level.
Its exon products have fully supported the establishment of the public database of whole exomes of the Han population under the "Huabiao Project" led by the team of Academician Jin Li from Fudan University. The pathogen detection kits have obtained EU CE certification.

Ten-Year Milestone: The Evolution of Technological Innovation
2014-2016: Tech Takes Root, Breaking Monopolies
Sep 2014: iGeneTech founded in Beijing, tackling key bottlenecks in gene capture technology.
Dec 2014: TargetSeq® hybrid capture platform launched, China’s first custom exome capture.
Tech breakthroughs: Two proprietary platforms filled domestic gaps.
2017-2019: Quality as Foundation, Scale Expands
May 2017: AIExome® V1 (human whole exome) launched, supporting Fudan University’s "Huabiao Project" to build Han population exome database.
Jan 2018: Earned ISO13485/9001 certifications, among China’s first gene capture firms meeting international standards.
May 2019: iGeneTech (Jiaxing) subsidiary established, with 2,000㎡ GMP production base for industrialization.
Industry recognition: Co-developed national standard GB/T 37872-2019 (Quality Evaluation for Target Gene Capture).
2020-2022: Sustained Efforts, Diversified Layout
Feb 2020: Amid the pandemic, developed β-coronavirus mutation monitoring kits in 6 days (launched during Lunar New Year), aiding Nature Communications publications and global anti-pandemic efforts.
May 2021: Launched RNA targeted sequencing solutions, boosting fusion gene detection sensitivity.
Jul 2022: TargetOne® v2.0 (RNA/DNA compatible, high stability) launched.
Aug 2022: AIExome® V3 released, covering >99.9% of CDS regions in major databases.
Nov 2022: Self-developed IGT-AS12 automated liquid handler, moving from manual to "unmanned lab" operations.
Honors: National High-Tech Enterprise, Beijing "Specialized & Sophisticated" SME, Zhongguancun High-Tech Enterprise, etc.
2023-2025: Global Vision, Leading the Future
Sep 2023: Restriction enzyme library prep kit V3 launched.
Jun 2024: Initiated free customization of MRD personalized Panels, advancing clinical use of tumor minimal residual disease.
Aug 2024: Co-developed national standard GB/T 44322-2024 (Forensic NGS Reagent Quality) released.
Jan 2025: Completed full-length probes for common pathogens in disease control; total probes exceeded 10 million.
Feb 2025: iGeneTech Quality Fundamental Regulation released.
Mar 2025: TargetOne® v3.0 and AIExome® V5 launched, reshaping the industry with superior uniformity and capture efficiency.
Apr 2025: "Capture Everything Initiative" upgraded, covering 20+ animal/plant whole exome products; total probes exceeded 10 million.
Jul 2025: Launched iGene Ignite 3.0 high-throughput oligo synthesis platform.
Sep 2025: To kick off iGeneTech’s brand building, with high-standard quality, co-creating the next decade’s growth blueprint with customers.
A Decade of Accumulation: Adherence to Three Core Principles
01 Technology as the Foundation: Over 30 patents build a moat
From the first-generation TargetSeq to AIExome® V5, we have accumulated 22 authorized invention patents and 9 utility model patents, forming "dry experiment design - wet experiment verification - automated production" full-chain capabilities.
02 Customer First: Growing with partners
100% of the intellectual property rights for customized Panels belong to customers; we provide 7×24 technical support with an average response time of < 2 hours; we have helped multiple customers publish high-level papers in Cell, Nature, Nature Medicine, etc.
03 Made-in-China Innovation: From "catching up" to "leading"
Products are exported to over 10 countries in Southeast Asia, Europe, etc., providing gene capture products and high-performance reagent components to nearly 1,000 customers.

 CN
CN








